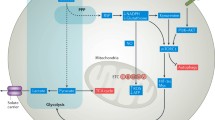
Abstract
Cellular metabolism represents a newly identified checkpoint of effector functions in the immune system. A solid body of work has characterized the metabolic requirements of normal T cells during activation and differentiation into polarized effector subsets. Similar studies have been initiated to characterize the metabolic requirements for B cells and myeloid cells. Only a few studies though have characterized the metabolism of immune cells in the context of autoimmune diseases. Here, we review what is known on the altered metabolic patterns of CD4+ T cells, B cells, and myeloid cells in lupus patients and lupus-prone mice and how they contribute to lupus pathogenesis. We also discuss how defects in immune metabolism in lupus can be targeted therapeutically.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–77.
Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–44.
Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212(9):1345–60.
O’Sullivan D, Pearce EL. Targeting T cell metabolism for therapy. Trends Immunol. 2015;36(2):71–80.
Powell JD, Pollizzi KN, Heikamp EB, et al. Regulation of immune responses by mTOR. Ann Rev Immunol. 2012;30(1):39–68.
MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Ann Rev Immunol. 2013;31:259–83. Comprehensive review of the basic mechanisms by which metabolism controls T cell effector functions.
Perl A, Gergely P, Puskas F, et al. Metabolic switches of T-cell activation and apoptosis. Antioxid Redox Signal. 2002;4(3):427–43.
Fernandez D, Perl A. Metabolic control of T cell activation and death in SLE. Autoimmun Rev. 2009;8(3):184–9.
Oaks Z, Perl A. Metabolic control of the epigenome in systemic lupus erythematosus. Autoimmunity. 2014;47(4):256–64. Review of the intersection between epigenetic regulation and cellular metabolism with a focus on T cells in SLE.
Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12(3):169–82. Comprehensive review of mTOR activition in rheumatic diseases with a focus on T cells, and its potential as therapeutic target.
Yokogami K, Wakisaka S, Avruch J, et al. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10(1):47–50.
Kim JH, Yoon MS, Chen J. Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J Biol Chem. 2009;284(51):35425–32.
Zeng H, Yang K, Cloer C, et al. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature. 2013;499:485–90.
Ray JP, Staron MM, Shyer JA, et al. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity. 2015;43(4):690–702. A comparision of Th1 and Tfh cells in virally infected mice showing a dichotomy in metabolism corresponding to the IL-2 and mTORC1 pathways.
Srivastava M, Duan G, Kershaw NJ, et al. Roquin binds microRNA-146a and Argonaute2 to regulate microRNA homeostasis. Nat Commun. 2015;6:6253.
Ramiscal RR, Parish IA, Lee-Young RS, et al. Attenuation of AMPK signaling by ROQUIN promotes T follicular helper cell formation. Elife. 2015;4:e08698. A study that showed that ROQUIN is a gene that regulates Tfh cell differentiation in a complex manner, including through direct inhibition of AMPK.
Pollizzi KN, Sun IH, Patel CH, et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol. 2016;17(6):704–11.
Verbist KC, Guy CS, Milasta S, et al. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532(7599):389–93.
Fernandez D, Perl A. mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov Med. 2010;9(46):173–8.
Lai ZW, Borsuk R, Shadakshari A, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol. 2013;191(5):2236–46. This study dissects the consequences of activated mTORC1 in human lupus T cells.
Kato H, Perl A. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4-CD8- double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol. 2014;192(9):4134–44. This study showed that the activation of mTORC1 has a selective effect on human lupus T cells.
Yin Y, Choi SC, Xu Z, et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7(274):274ra18. This study showed that a treatment combining metformin and a glucose inhibitor reversed disease in lupus-prone mice and normalised the metabolism of their CD4+ T cells.
Yin Y, Choi S-C, Xu Z, et al. Glucose oxidation is critical for CD4+ T cell activation in a mouse model of systemic lupus erythematosus. J Immunol. 2016;196(1):80–90. This study showed that murine lupus T cell oxidizes glucose, identifying mitochondrial metabolism as the major mechanism to sustain their activation.
Lai ZW, Hanczko R, Bonilla E, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–46.
Fernandez D, Bonilla E, Mirza N, et al. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2983–8.
Lui SL, Tsang R, Chan KW, et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol Dial Transplant. 2008;23(9):2768–76.
Oaks Z, Winans T, Caza T, et al. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis & Rheumatol. 2016: Jun 22. doi: 10.1002/art.39791. [Epub ahead of print].
Macintyre AN, Gerriets VA, Nichols AG, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20(1):61–72. This study established that glucose uptake through Glut1 is essential for CD4+ T cell activation.
Jacobs SR, Herman CE, MacIver NJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180(7):4476–86.
Maekawa Y, Ishifune C, Tsukumo S, et al. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med. 2015;21(1):55–61.
Yang ZC, Liu Y. Hypoxia-inducible factor-1alpha and autoimmune lupus, arthritis. Inflammation. 2016;39(3):1268–73.
Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–38.
Shi LZ, Wang R, Huang G, et al. HIF1α–dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208(7):1367–76.
Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol. 2016;12(3):143–53.
Kolev M, Dimeloe S, Le Friec G, et al. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell response. Immunity. 2015;42(6):1033–47. This study revealed a complex role for complement receptor CD46 in promoting Th1 differentiation through metabolic reprogramming.
Le Buanec H, Gougeon ML, Mathian A, et al. IFN-alpha and CD46 stimulation are associated with active lupus and skew natural T regulatory cell differentiation to type 1 regulatory T (Tr1) cells. Proc Natl Acad Sci U S A. 2011;108(47):18995–9000.
Wahl DR, Petersen B, Warner R, et al. Characterization of the metabolic phenotype of chronically activated lymphocytes. Lupus. 2010;19(13):1492–501.
Glick GD, Rossignol R, Lyssiotis CA, et al. Anaplerotic metabolism of alloreactive T cells provides a metabolic approach to treat graft-versus-host disease. J Pharmacol Exp Ther. 2014;351(2):298–307.
Nguyen HD, Chatterjee S, Haarberg KM, et al. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest. 2016;126(4):1337–52.
Doherty E, Oaks Z, Perl A. Increased mitochondrial electron transport chain activity at complex i is regulated by N-Acetylcysteine in lymphocytes of patients with systemic lupus erythematosus. Antioxid Redox Signal. 2014;21(1):56–65.
Dimeloe S, Mehling M, Frick C, et al. The immune-metabolic basis of effector memory CD4+ T cell function under hypoxic conditions. J Immunol. 2016;196(1):106–14.
Perl A, Gergely Jr P, Nagy G, et al. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25(7):360–7.
Sobel ES, Brusko TM, Butfiloski EJ, et al. Defective response of CD4+ T cells to retinoic acid and TGFbeta in systemic lupus erythematosus. Arthritis ResTher. 2011;13(3):R106.
Morel L, Croker BP, Blenman KR, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A. 2000;97(12):6670–5.
Bricker DK, Taylor EB, Schell JC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100.
Herzig S, Raemy E, Montessuit S, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337(6090):93–6.
Divakaruni AS, Wiley SE, Rogers GW, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110(14):5422–7.
Colca JR, McDonald WG, Cavey GS, et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT): relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS One. 2013;8(5), e61551.
Zhao W, Berthier CC, Lewis EE, et al. The peroxisome-proliferator activated receptor-γ agonist pioglitazone modulates aberrant T cell responses in systemic lupus erythematosus. Clin Immunol. 2013;149(1):119–32.
Aprahamian T, Bonegio RG, Richez C, et al. The peroxisome proliferator-activated receptor γ agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J Immunol. 2009;182(1):340–6.
Teruel M, Alarcon-Riquelme ME. Genetics of systemic lupus erythematosus and Sjogren’s syndrome: an update. Curr Opin Rheumatol. 2016;28(5):506–14.
X-j Z, Lu X-l, J-c L, et al. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis. 2011;70(7):1330–7.
Lessard CJ, Sajuthi S, Zhao J, et al. Identification of a systemic lupus erythematosus risk locus spanning ATG16L2, FCHSD2, and P2RY2 in koreans. Arthritis Rheumatol. 2016;68(5):1197–209.
Martinez J, Cunha LD, Park S, et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533(7601):115–9. A novel role for Atg5 in regulating apototic cell clearance by myeloid cells through non-canonical autophagy.
Ishibashi K, Fujita N, Kanno E, et al. Atg16L2, a novel isoform of mammalian Atg16L that is not essential for canonical autophagy despite forming an Atg12-5-16L2 complex. Autophagy. 2011;7(12):1500–13.
Rockel JS, Kapoor M. Autophagy: controlling cell fate in rheumatic diseases. Nat Rev Rheumatol. 2016;12(9):517–31.
Liszewski MK, Atkinson JP. Complement regulator CD46: genetic variants and disease associations. Human Genomics. 2015;9(1):1–13.
Perry DJ, Yin Y, Telarico T, et al. Murine lupus susceptibility locus Sle1c2 mediates CD4+ T cell activation and maps to estrogen-related receptor gamma. J Immunol. 2012;189(2):793–803.
Eichner LJ, Giguere V. Estrogen related receptors (ERRs): a new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion. 2011;11(4):544–52.
Alaynick WA, Kondo RP, Xie W, et al. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6(1):13–24.
Yoshihara E, Wei Z, Lin CS, et al. ERRgamma is required for the metabolic maturation of therapeutically functional glucose-responsive beta cells. Cell Metab. 2016;23(4):622–34.
Pei L, Mu Y, Leblanc M, et al. Dependence of hippocampal function on ERRgamma-regulated mitochondrial metabolism. Cell Metab. 2015;21(4):628–36.
Kida YS, Kawamura T, Wei Z, et al. ERRs mediate a metabolic switch required for somatic cell reprogramming to pluripotency. Cell Stem Cell. 2015;16(5):547–55.
Michalek RD, Gerriets VA, Nichols AG, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci U S A. 2011;108(45):18348–53.
Murray PJ, Rathmell J, Pearce E. SnapShot: immunometabolism. Cell Metab. 2015;22(1):190–90.e1.
Caro-Maldonado A, Wang R, Nichols AG, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol. 2014;192(8):3626–36. This study showed that elevated levels of BAFF push B cells through a highly glycolytic metabolism associated with higher effector functions.
Cho SH, Raybuck AL, Stengel K, et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;advance online publication. A novel mechanism regulating B cell functions through a graded level of hypoxia in germinal centers.
Aronov M, Tirosh B. Metabolic control of plasma cell differentiation—what we know and what we don’t know. J Clin Immunol. 2016;36 Suppl 1:12–7.
Lam WY, Becker AM, Kennerly KM, et al. Mitochondrial pyruvate import promotes long-term survival of antibody-secreting plasma cells. Immunity. 2016;45(1):60–73. An elegant study showing that pyruvate oxidation is required by plasma cells to be long-lived.
Pengo N, Scolari M, Oliva L, et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat Immunol. 2013;14(3):298–305.
Clarke AJ, Ellinghaus U, Cortini A, et al. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann Rheum Dis. 2015;74(5):912–20. This study implicated autophagy as promoting the survival and activation of autoreactive B cells in mice.
Arnold J, Murera D, Arbogast F, et al. Autophagy is dispensable for B-cell development but essential for humoral autoimmune responses. Cell Death Differ. 2016;23(5):853–64.
Benhamron S, Pattanayak SP, Berger M, et al. mTOR activation promotes plasma cell differentiation and bypasses XBP-1 for immunoglobulin secretion. Mol Cell Biol. 2015;35(1):153–66.
Pearce EJ, Everts B. Dendritic cell metabolism. Nat Rev Immunol. 2015;15(1):18–29.
O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213(1):15–23.
Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420.
Cao W, Manicassamy S, Tang H, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9(10):1157–64.
Boor PP, Metselaar HJ, Mancham S, et al. Rapamycin has suppressive and stimulatory effects on human plasmacytoid dendritic cell functions. Clin Exp Immunol. 2013;174(3):389–401.
Bao Y, Ledderose C, Graf AF, et al. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J Cell Biol. 2015;210(7):1153–64.
Donnelly RP, Loftus RM, Keating SE, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193(9):4477–84.
Keating SE, Zaiatz-Bittencourt V, Loftus RM, et al. Metabolic reprogramming supports IFN-gamma production by CD56bright NK cells. J Immunol. 2016;196(6):2552–60.
Yang Z, Shen Y, Oishi H, et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016;8(331):331–8.
Gatza E, Wahl DR, Opipari AW, et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med. 2011;3(67):67ra8–8.
Shirai T, Nazarewicz RR, Wallis BB, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213(3):337–54.
Lee CF, Lo YC, Cheng CH, et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep. 2015;13(4):760–70.
Kishton RJ, Rathmell JC. Novel therapeutic targets of tumor metabolism. Cancer J. 2015;21(2):62–9.
Yang Z, Shen Y, Oishi H, et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016;8(331):331ra38. This study dissected the abormal metabolism of T cells from RA patients, and showed that it can be normalized with oxidizing drugs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Additional information
This article is part of the Topical Collection on Systemic Lupus Erythematosus
Rights and permissions
About this article
Cite this article
Choi, SC., Titov, A.A., Sivakumar, R. et al. Immune Cell Metabolism in Systemic Lupus Erythematosus. Curr Rheumatol Rep 18, 66 (2016). https://doi.org/10.1007/s11926-016-0615-7
Published:
DOI: https://doi.org/10.1007/s11926-016-0615-7