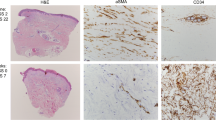
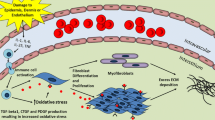
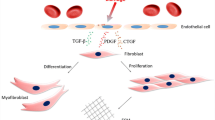
Abstract
Skin fibrosis is the final outcome of a variety of pathologic processes ranging from aberrant wound healing (keloids) to environmentally induced conditions (nephrogenic systemic fibrosis) to idiopathic or autoimmune conditions (morphea and systemic sclerosis). The quantitative assessment of skin fibrosis has been a major burden of clinical and biomarker research in the field for the past three decades. Here, we review the efforts that reached some sort of validation and the ones we envisage have the potential for further development focusing on systemic sclerosis as prototype of fibrotic disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wick G, Grundtman C, Mayerl C, et al. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107–35.
Steen VD, Medsger Jr TA. Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 2001;44:2828–35.
Clements PJ, Hurwitz EL, Wong WK, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000;43:2445–54.
LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5.
Rodnan GP, Lipinski E, Luksick J. Skin thickness and collagen content in progressive systemic sclerosis and localized scleroderma. Arthritis Rheum. 1979;22:130–40.
Czirjak L, Foeldvari I, Muller-Ladner U. Skin involvement in systemic sclerosis. Rheumatology (Oxford). 2008;47:v44–5.
Clements PJ, Furst DE, Wong WK, et al. High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum. 1999;42:1194–203.
Khanna D, Clements PJ, Furst DE, et al. Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:1102–11.
Merkel PA, Clements PJ, Reveille JD, et al. Current status of outcome measure development for clinical trials in systemic sclerosis: report from OMERACT 6. J Rheumatol. 2003;30:1630–47.
Pope JE, Baron M, Bellamy N, et al. Variability of skin scores and clinical measurements in scleroderma. J Rheumatol. 1995;22:1271–6.
Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–6.
Clements PJ. Measuring disease activity and severity in scleroderma. Curr Opin Rheumatol. 1995;7:517–21.
Falanga V, Bucalo B. Use of a durometer to assess skin hardness. J Am Acad Dermatol. 1993;29:47–51.
Kissin EY, Schiller AM, Gelbard RB, et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006;55:603–9.
Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum. 2006;54:3655–60.
Merkel PA, Silliman NP, Denton CP, for the CAT-192 Research Group and the Scleroderma Clinical Trials Consortium, et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum. 2008;59:699–705.
Nives Parodi M, Castagneto C, Filaci G, et al. Plicometer skin test: a new technique for the evaluation of cutaneous involvement in systemic sclerosis. Br J Rheumatol. 1997;36:244–50.
Kuwahara Y, Shima Y, Shirayama D, et al. Quantification of hardness, elasticity and viscosity of the skin of patients with systemic sclerosis using a novel sensing device (Vesmeter): a proposal for a new outcome measurement procedure. Rheumatology (Oxford). 2008;47:1018–24.
Enomoto DN, Mekkes JR, Bossuyt PM, et al. Quantification of cutaneous sclerosis with a skin elasticity meter in patients with generalized scleroderma. J Am Acad Dermatol. 1996;35:381–7.
Black MM, Bottoms E, Shuster S. Skin collagen content and thickness in systemic sclerosis. Br J Dermatol. 1970;83:552–5.
Castro S, Jimenez SA. Biomarkers in systemic sclerosis. Biomark Med. 2010;4:133–47.
Abignano G, Buch M, Emery P, et al. Biomarkers in the management of scleroderma: an update. Curr Rheumatol Rep. 2011;13:4–12.
Moinzadeh P, Denton CP, Abraham D, et al. Biomarkers for skin involvement and fibrotic activity in scleroderma. J Eur Acad Dermatol Venereol. 2012;26:267–76. This review, analyzing the biomarkers for skin involvement in systemic sclerosis developed to date, gives a wide view of the advances in this field highlighting the weaknesses and strengths of each.
Castelino FV, Varga J. Current status of systemic sclerosis biomarkers: applications for diagnosis, management and drug development. Expert Rev Clin Immunol. 2013;9:1077–90.
Hesselstrand R, Kassner A, Heinegård D, et al. COMP: a candidate molecule in the pathogenesis of systemic sclerosis with a potential as a disease marker. Ann Rheum Dis. 2008;67:1242–8.
Hesselstrand R, Andréasson K, Wuttge DM, et al. Increased serum COMP predicts mortality in SSc: results from a longitudinal study of interstitial lung disease. Rheumatology (Oxford). 2012;51:915–20. This study recently showed that serum COMP is a predictor of mortality in systemic sclerosis, and is a stronger marker of skin rather than lung fibrosis.
Farina G, Lemaire R, Korn JH, et al. Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts. Matrix Biol. 2006;25:213–22.
Farina G, Lemaire R, Pancari P, et al. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann Rheum Dis. 2009;68:435–41.
Farina G, Lafyatis D, Lemaire R, et al. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62:580–8.
Abignano G, Cuomo G, Buch MH, et al. ELF TEST: a clinical grade, validated serum test, biomarker of overall fibrosis in systemic sclerosis. Ann Rheum Dis. 2013;0:1–8. doi:10.1136/annrheumdis-2012-202843. This study shows the potential use of three CE-marked serum tests as surrogate outcome measures of overall fibrosis in scleroderma.
Chapin R, Hant FN. Imaging of scleroderma. Rheum Dis Clin N Am. 2013;39:515–46.
Akesson A, Hesselstrand R, Scheja A, et al. Longitudinal development of skin involvement and reliability of high frequency ultrasound in systemic sclerosis. Ann Rheum Dis. 2004;63:791–6.
Kaloudi O, Bandinelli F, Filippucci E, et al. High frequency ultrasound measurement of digital dermal thickness in systemic sclerosis. Ann Rheum Dis. 2010;69:1140–3.
Ch'ng SS, Roddy J, Keen HI. A systematic review of ultrasonography as an outcome measure of skin involvement in systemic sclerosis. Int J Rheum Dis. 2013;16:264–72. This is a comprehensive review of the most important studies published between 1995 and 2010 regarding the use of ultrasonography as an outcome measure of skin involvement in systemic sclerosis. It also highlights the current unmet need and the major aspects to be developed in further studies.
Ihn H, Shimozuma M, Fujimoto M, et al. Ultrasound measurement of skin thickness in systemic sclerosis. Br J Rheumatol. 1995;34:535–8.
Hesselstrand R, Scheja A, Wildt M, et al. High-frequency ultrasound of skin involvement in systemic sclerosis reflects oedema, extension and severity in early disease. Rheumatology. 2008;47:84–7.
Iagnocco A, Kaloudi O, Perella C, et al. Ultrasound elastography assessment of skin involvement in systemic sclerosis: lights and shadows. J Rheumatol. 2010;37:1688–91.
Di Geso L, Filippucci E, Girolimetti R, et al. Reliability of ultrasound measurements of dermal thickness at digits in systemic sclerosis: role of elastosonography. Clin Exp Rheumatol. 2011;29:926–32.
Santiago T, Alcacer-Pitarch B, Del Galdo F, et al. Assessment of skin involvement by acoustic radiation force impulse (ARFI) imaging in patients with systemic sclerosis. Ann Rheum Dis. 2013;72(Suppl3):157. Although preliminary, this study reveals the potential of shear wave ultrasound in quantifying skin fibrosis in scleroderma.
Schmitt AM. Principles and application of optical coherent tomography in dermatology. Dermatology. 2008;217:12–3.
Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81.
Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457–73.
Marschall S, Sander B, Mogensen M, Jørgensen TM, Andersen PE. Optical coherence tomography-current technology and applications in clinical and biomedical research. Anal Bioanal Chem. 2011;400:2699–720.
Welzel J, Lankenau E, Birngruber R, et al. Optical coherence tomography of the human skin. J Am Acad Dermatol. 1997;37:958–63.
Chu CR, Williams A, Tolliver D, et al. Clinical optical coherence tomography of early articular cartilage degeneration in patients with degenerative meniscal tears. Arthritis Rheum. 2010;62:1412–20.
Aydin SZ, Ash Z, Del Galdo F, et al. Optical coherence tomography: a new tool to assess nail disease in psoriasis? Dermatology. 2011;222:311–3.
Aydin SZ, Castillo-Gallego C, Ash ZR, et al. Potential use of optical coherence tomography and high-frequency ultrasound for the assessment of nail disease in psoriasis and psoriatic arthritis. Dermatology. 2013. doi:10.1159/000351702.
Babalola O, Mamalis A, Lev-Tov H, et al. Optical coherence tomography (OCT) of collagen in normal skin and skin fibrosis. Arch Dermatol Res. 2013. doi:10.1007/s00403-013-1417-7. The authors review the most important studies involving optical coherence tomography and sketch the potential development of the technique in skin imaging.
Mogensen M, Morsy HA, Thrane L, et al. Morphology and epidermal thickness of normal skin imaged by optical coherence tomography. Dermatology. 2008;217:14–20.
Abignano G, Aydin SZ, Castillo-Gallego C, et al. Virtual skin biopsy by optical coherence tomography: the first quantitative imaging biomarker for scleroderma. Ann Rheum Dis. 2013;72:1845–51. This is the first study validating optical coherence tomography as a quantitative and reliable biomarker of severity of skin involvement in systemic sclerosis.
Alex A, Povazay B, Hofer B, et al. Multispectral in vivo three-dimensional optical coherence tomography of human skin. J Biomed Opt. 2010;15:026025.
Gladkova ND, Petrova GA, Nikulin NK, et al. In vivo optical coherence tomography imaging of human skin: norm and pathology. Skin Res Technol. 2000;6:6–16.
Gambichler T, Matip R, Moussa G, Altmeyer P, Hoffmann K. In vivo data of epidermal thickness evaluated by optical coherence tomography: effects of age, gender, skin type, and anatomic site. J Dermatol Sci. 2006;44:145–52.
Gambichler T, Boms S, Stücker M, Kreuter A, Sand M, Moussa G, et al. Comparison of histometric data obtained by optical coherence tomography and routine histology. J Biomed Opt. 2005;10:44008.
Gambichler T, Moussa G, Regeniter P, Kasseck C, Hofmann MR, Bechara FG, et al. Validation of optical coherence tomography in vivo using cryostat histology. Phys Med Biol. 2007;52:N75–85.
Compliance with Ethics Guidelines
Conflict of Interest
Giuseppina Abignano and Francesco Del Galdo declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Abignano, G., Del Galdo, F. Quantitating Skin Fibrosis: Innovative Strategies and Their Clinical Implications. Curr Rheumatol Rep 16, 404 (2014). https://doi.org/10.1007/s11926-013-0404-5
Published:
DOI: https://doi.org/10.1007/s11926-013-0404-5