Abstract
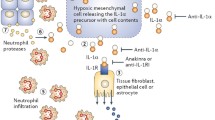
Gout is the most common inflammatory arthritis in humans. Current treatment options to control the pain and inflammation of acute and chronic gout include nonsteroidal anti-inflammatory drugs, colchicine, and corticosteroids. However, patients are commonly unresponsive to, intolerant of, or have contraindications to current treatments. Interleukin-1 (IL-1), a proinflammatory cytokine, plays a major role in mediating gouty inflammation. This role of IL-1 has led investigators to explore a new class of anti-inflammatory drugs that inhibit IL-1 signal transduction. IL-1 inhibitors currently in trials for gout include anakinra, rilonacept, and canakinumab. Anakinra is an IL‑1 receptor antagonist that inhibits the activity of both IL‑1α and IL‑1β, rilonacept is a soluble decoy receptor and canakinumab is an anti‑IL‑1β monoclonal antibody. In case cohorts, anakinra was found to be efficacious in combating acute gout pain and inflammation, whereas rilonacept has been found to be efficacious for reducing the risk of recurrent attacks. Canakinumab has been shown to be efficacious in both reducing pain and inflammation in acute attacks, and for reducing the risk of recurrent attacks. All three IL-1 inhibitors are generally well tolerated. This article reviews the current IL-1 inhibitors and the results of trials in which they have been tested for the management of acute and chronic gouty inflammation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. This is an excellent paper addressing incidence of frequency of gout in the USA.
• Keenan RT, O'Brien WR, Lee KH, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011;124:155–63. This is an excellent paper addressing much needed information about comorbidities in gout and how they affect prescriptions.
Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–71.
Di Giovine FS, Malawista SE, Nuki G, Duff GW. Interleukin 1 (IL 1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal- induced IL-1. J Immunol. 1987;138:3213–8.
Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41.
Cronstein RN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8 Suppl 1:S3.
So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28.
Cho M, Ghosh P, Hans G, Rhiannon J, Gardner GC, Simkin PA. The safety and efficacy of anakinra in the treatment of acute gout in hospitalized patients. Arthritis Rheum. 2010;60(9, Supplement):S163.
Chen K, Fields T, Mancuso CA, Bass AR, Vasanth L. Anakinra's efficacy is variable in refractory gout: report of ten cases. Semin Arthritis Rheum. 2010;40:210–4.
Ottaviani S, Moltò A, Ea H-K, Neveu S, Gill G, Brunier L, et al. Efficacy of anakinra in gouty arthritis: a retrospective study of 40 cases. Arthritis Res Ther. 2013;15:R123.
propertieshttp://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
Kapur S, Bonk ME. Rilonacept (Arcalyst), an Interleukin-1 Trap for the Treatment of Cryopyrin-Associated Periodic Syndromes. P T. 2009;34(3):138–41.
Terkeltaub RA, Schumacher HR, Carter JD, Baraf HSB, Evans RR, Wang J, et al. Rilonacept in the treatment of acute gouty arthritis: a randomized, controlled clinical trial using indomethacin as the active comparator. Arthritis Res Ther. 2013;15(1):R25.
Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, Bookbinder S, Biedermann S, et al. The interleukin 1 inhibitor rilonacept in the treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68:1613–7.
Mitha E, Schumacher HR, Fouche L, Luo SF, Weinstein SP, Yancopoulos GD, et al. Rilonacept for gout flare prevention during initiation of uric acid-lowering therapy: results from the PRESURGE-2 international, phase 3, randomized, placebo-controlled trial. Rheumatology. 2013;52(7):1285–92.
•• Schumacher HR, Sundy JS, Terkeltaub R, Knapp HR, Mellis SJ, Stahl N, et al. Rilonacept (IL-1 Trap) in the prevention of acute gout flares during initiation of urate-lowering therapy: results of a phase 2 clinical trial. Arthritis Rheum. 2012;64(3):876–84. An important study demonstrating the use of IL-1 blockade in gout prophylaxis.
So A, De Meulemeester M, Pikhlak A, Yücel AE, Richard D, Murphy V, et al. Canakinumab for treatment of acute flares in refractory gouty arthritis. Arthritis Rheum. 2010;62(10):3064–74. PA.
Schlesinger N, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat Gouty Arthritis by suppressing inflammation: results of a randomized, dose-ranging study. Arthritis Res Ther. 2011;13:R53.
•• Schlesinger N, Mysler E, Lin HY, De Meulemeester M, Rovensky J, Arulmani U, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol therapy: results of a double-blind, randomised study. Ann Rheum Dis. 2011;70(7):1264–71. An important study demonstrating the use of IL-1 blockade in gout prophylaxis.
Schlesinger N, Alten R, Bardin T, Schumacher HR, Bloch M, Gimona A, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their extensions. Ann Rheum Dis. 2012;71:1839–48.
de La Mata Llord J, Gonzalez Crespo R, Maese Manzano J. Treatment of rheumatoid arthritis with anakinra: a systematic review. Reumatol Clin. 2007;3(4):153–8.
Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25–32.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Naomi Schlesinger has received grant support from Novartis; has served on advisory boards for Novartis, Takeda, Savient, Enzyme Rx, URL Pharma, and Sobi; has served as a consultant for Novartis and Sobi; and has served on speakers bureaus for Novartis, Takeda, and Savient.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Crystal Arthritis
Rights and permissions
About this article
Cite this article
Schlesinger, N. Anti-Interleukin-1 Therapy in the Management of Gout. Curr Rheumatol Rep 16, 398 (2014). https://doi.org/10.1007/s11926-013-0398-z
Published:
DOI: https://doi.org/10.1007/s11926-013-0398-z