Abstract
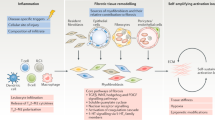
The morphogen pathways Wnt, hedgehog, and Notch are key regulators of organ development and tissue homeostasis. In adults, the tightly regulated activity of morphogen pathways is essential for cell renewal and tissue regeneration. Loss of control and persistent activation of morphogen pathways, however, can lead to a variety of diseases, including malignancy and fibrotic disorders. In recent years, pathological activation of Wnt, hedgehog, and Notch pathways have been described in systemic sclerosis (SSc) and other fibrotic diseases. Experimental models reveal that morphogen pathways drive fibroblast activation and collagen release. In these model systems, genetic or pharmacological blockade of morphogen pathways inhibits collagen release and reduces experimental fibrosis. Importantly, inhibitors for Wnt, hedgehog, and Notch are already in clinical evaluation, thereby emphasizing the translational implications of these findings. Further experimental studies, however, should deepen our knowledge before initiating clinical trials with inhibitors of morphogen pathways.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–67.
Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989–2003.
Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66(7):940–4.
Beyer C, Distler O, Distler JH. Innovative antifibrotic therapies in systemic sclerosis. Curr Opin Rheumatol. 2012;24(3):274–80.
Beyer C, Distler JH. The scientific basis for novel treatments of systemic sclerosis. F1000 Med Rep. 2009;1:95.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
• Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31(12):2670–84. This insightful review on Wnt signaling highlights the key steps in the Wnt pathway and how they have been elucidated. An excellent paper to first enter the field of Wnt signaling.
Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391(6666):493–6.
Maniatis T. A ubiquitin ligase complex essential for the NF-kappaB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev. 1999;13(5):505–10.
Yang L, Lin C, Liu ZR. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell. 2006;127(1):139–55.
Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31(12):2714–36.
•• Akhmetshina A, Palumbo K, Dees C, et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. This paper explores the important link between the two pro-fibrotic pathways canonical Wnt and TGFβ-signaling. The study demonstrates that canonical Wnt-signaling is induced by TGFβ in fibrotic disease, and promotes the progression of fibrosis.
He W, Dai C, Li Y, et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20(4):765–76.
Konigshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–87.
Kobayashi K, Luo M, Zhang Y, et al. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol. 2009;11(1):46–55.
Beyer C, Schramm A, Akhmetshina A, et al. beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis. 2012;71(5):761–7.
Lam AP, Flozak AS, Russell S, et al. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. 2011;45(5):915–22.
• Wei J, Melichian D, Komura K, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63(6):1707–17. Wei and colleagues are the first to show that canonical Wnt signaling promotes dermal fibrosis in SSc.
Wei J, Fang F, Lam AP, et al. Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012.
Bayle J, Fitch J, Jacobsen K, et al. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128(4):871–81.
Bergmann C, Akhmetshina A, Dees C, et al. Inhibition of glycogen synthase kinase 3beta induces dermal fibrosis by activation of the canonical Wnt pathway. Ann Rheum Dis. 2011;70(12):2191–8.
Longo KA, Wright WS, Kang S, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279(34):35503–9.
Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31(12):2737–46.
Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145(6):851–62.
Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5(2):100–7.
Fujii N, You L, Xu Z, et al. An antagonist of dishevelled protein–protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res. 2007;67(2):573–9.
Huang SM, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20.
Lepourcelet M, Chen YN, France DS, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5(1):91–102.
Hao S, He W, Li Y, et al. Targeted Inhibition of {beta}-Catenin/CBP Signaling Ameliorates Renal Interstitial Fibrosis. J Am Soc Nephrol. 2011.
Henderson Jr WR, Chi EY, Ye X, et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107(32):14309–14.
Distler A, Deloch L, Huang J, et al. Inactivation of tankyrases inhibits canonical Wnt signaling and reduces experimental fibrosis. Ann Rheum Dis. 2011;submitted.
Beyer C, Schramm A, Akan H, et al. Blockade of canonical Wnt signaling inhibits experimental dermal fibrosis. Ann Rheum Dis. 2012;submitted.
Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12(6):393–406.
Gallet A. Hedgehog morphogen: from secretion to reception. Trends Cell Biol. 2011;21(4):238–46.
Horn A, Palumbo K, Cordazzo C, et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 2012.
Horn A, Kireva T, Palumbo-Zerr K, et al. Inhibition of hedgehog signalling prevents experimental fibrosis and induces regression of established fibrosis. Ann Rheum Dis. 2012;71(5):785–9.
Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43(2):238–44.
• Zerr P, Palumbo-Zerr K, Akhmetshina A, et al. Inhibition of hedgehog signaling for the treatment of murine sclerodermatous chronic graft-versus-host disease. Blood. 2012;120(14):2909–17. The fibrotic disease manifestations of sclerodermatous chronic GvHD closely resemble those of diffuse-cutaneous SSc. This paper shows that inhibition of hedgehog signaling is effective in treating experimental sclGvHD without reducing the desired graft-versus-lymphoma effect.
Lear JT. Oral hedgehog-pathway inhibitors for basal-cell carcinoma. N Engl J Med. 2012;366(23):2225–6.
Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16(5):633–47.
Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138(17):3593–612.
Dees C, Tomcik M, Zerr P, et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis. 2011;70(7):1304–10.
• Kavian N, Servettaz A, Mongaret C, et al. Targeting ADAM-17/notch signaling abrogates the development of systemic sclerosis in a murine model. Arthritis Rheum. 2010;62(11):3477–87. Kavian et al. are the first to demonstrate anti-fibrotic effects of Notch inhibition in an inflammatory model of SSc fibrosis.
Dees C, Zerr P, Tomcik M, et al. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 2011;63(5):1396–404.
Sirin Y, Susztak K. Notch in the kidney: development and disease. J Pathol. 2012;226(2):394–403.
Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science. 1996;271(5257):1826–32.
Blokzijl A, Dahlqvist C, Reissmann E, et al. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol. 2003;163(4):723–8.
Dahlqvist C, Blokzijl A, Chapman G, et al. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130(24):6089–99.
Itoh F, Itoh S, Goumans MJ, et al. Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. EMBO J. 2004;23(3):541–51.
Imbimbo BP, Giardina GA. gamma-secretase inhibitors and modulators for the treatment of Alzheimer’s disease: disappointments and hopes. Curr Top Med Chem. 2011;11(12):1555–70.
Acknowledgments
Dr Beyer is a research scholar at the Interdisciplinary Center of Clinical Research in Erlangen (“Erstantragsstellerprogramm”). Dr Distler is the recipient of grant A40 of the Interdisciplinary Center of Clinical Research (IZKF) in Erlangen, grants from the Deutsche Forschungsgesellschaft, and the Career Support Award of Medicine of the Ernst Jung Foundation.
Disclosure
Dr Distler has served on boards for Celgene Corp., Bayer, and JB Therapeutics; has served as a consultant and received honoraria from Celgene Corp., Bayer, JB Therapeutics, Boehringer Ingelheim GmbH, BioPharma, Active Biotech, Actelion Pharmaceuticals Ltd., Pfizer, Ergonex Pharma GmbH, and Bristol-Myers Squibb; has received payment for development of educational presentations (including service on speakers’ bureaus) from Celgene Corp., Bayer, JB Therapeutics, Boehringer Ingelheim GmbH, BioPharma, Active Biotech, Actelion Pharmaceuticals Ltd., Pfizer, Ergonex Pharma GmbH, Roche, and Bristol-Myers Squibb; and has held stock/stock options from 4D Science GmbH (a company by which his wife has also been employed). Dr Beyer reported no potential conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Beyer, C., Distler, J.H.W. Morphogen Pathways in Systemic Sclerosis. Curr Rheumatol Rep 15, 299 (2013). https://doi.org/10.1007/s11926-012-0299-6
Published:
DOI: https://doi.org/10.1007/s11926-012-0299-6