Abstract
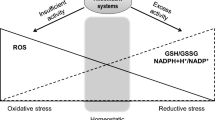
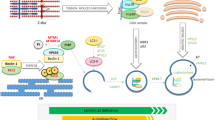
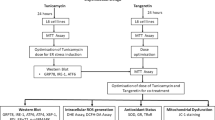
Our appreciation of the role of endoplasmic reticulum (ER) stress pathways in both skeletal muscle homeostasis and the progression of muscle diseases is gaining momentum. This review provides insight into ER stress mechanisms during physiologic and pathological disturbances in skeletal muscle. The role of ER stress in the response to dietary alterations and acute stressors, including its role in autoimmune and genetic muscle disorders, has been described. Recent studies identifying ER stress markers in diseased skeletal muscle are noted. The emerging evidence for ER–mitochondrial interplay in skeletal muscle and its importance during chronic ER stress in activation of both inflammatory and cell death pathways (autophagy, necrosis, and apoptosis) have been discussed. Thus, understanding the ER stress–related molecular pathways underlying physiologic and pathological phenotypes in healthy and diseased skeletal muscle should lead to novel therapeutic targets for muscle disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–33.
Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–4.
Matsumoto M, Minami M, Takeda K, Sakao Y, Akira S. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 1996;395:143–7.
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103.
Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17.
Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev. 2011;91:1219–43.
Ikezoe K, Furuya H, Ohyagi Y, Osoegawa M, Nishino I, et al. Dysferlin expression in tubular aggregates: their possible relationship to endoplasmic reticulum stress. Acta Neuropathol. 2003;105:603–9.
Ikezoe K, Nakamori M, Furuya H, Arahata H, Kanemoto S, et al. Endoplasmic reticulum stress in myotonic dystrophy type 1 muscle. Acta Neuropathol. 2007;114:527–35.
Vattemi G, Engel WK, McFerrin J, Askanas V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol. 2004;164:1–7.
Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52:1824–35.
Deldicque L, Hespel P, Francaux M (2011) ER stress in skeletal muscle: origin and metabolic consequences. Exerc Sport Sci Rev.
Deldicque L, Cani PD, Philp A, Raymackers JM, Meakin PJ, et al. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am J Physiol Endocrinol Metab. 2010;299:E695–705.
Deldicque L, Bertrand L, Patton A, Francaux M, Baar K. ER stress induces anabolic resistance in muscle cells through PKB-induced blockade of mTORC1. PLoS One. 2011;6:e20993.
• Kim HJ, Jamart C, Deldicque L, An GL, Lee YH, et al. (2011) Endoplasmic reticulum stress markers and ubiquitin-proteasome pathway activity in response to a 200-km run. Med Sci Sports Exerc. 43:18–25. This article demonstrates that ER stress pathways are enhanced in skeletal muscle in response to long-distance running.
• Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, et al. (2011) The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metab. 13:160–169. This article highlights the importance of the PGC-1α/ATF6α complex in the adaptation of skeletal muscle to exercise.
• Vitadello M, Doria A, Tarricone E, Ghirardello A, Gorza L (2010) Myofiber stress-response in myositis: parallel investigations on patients and experimental animal models of muscle regeneration and systemic inflammation. Arthritis Res Ther. 12:R52. This article substantiates that ER stress pathways are enhanced in the myositis muscle.
Nogalska A, Engel WK, McFerrin J, Kokame K, Komano H, et al. Homocysteine-induced endoplasmic reticulum protein (Herp) is up-regulated in sporadic inclusion-body myositis and in endoplasmic reticulum stress-induced cultured human muscle fibers. J Neurochem. 2006;96:1491–9.
Kokame K, Agarwala KL, Kato H, Miyata T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem. 2000;275:32846–53.
Chan SL, Fu W, Zhang P, Cheng A, Lee J, et al. Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. J Biol Chem. 2004;279:28733–43.
Delaunay A, Bromberg KD, Hayashi Y, Mirabella M, Burch D, et al. The ER-bound RING finger protein 5 (RNF5/RMA1) causes degenerative myopathy in transgenic mice and is deregulated in inclusion body myositis. PLoS One. 2008;3:e1609.
Tournadre A, Lenief V, Miossec P Expression of Toll-like receptor 3 and Toll-like receptor 7 in muscle is characteristic of inflammatory myopathy and is differentially regulated by Th1 and Th17 cytokines. Arthritis Rheum. 62:2144–2151.
Pandya JM, Fasth AE, Zong M, Arnardottir S, Dani L, et al. Expanded T cell receptor Vbeta-restricted T cells from patients with sporadic inclusion body myositis are proinflammatory and cytotoxic CD28null T cells. Arthritis Rheum. 62:3457–3466.
Fasth AE, Dastmalchi M, Rahbar A, Salomonsson S, Pandya JM, et al. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J Immunol. 2009;183:4792–9.
Rayavarapu S, Coley W, Nagaraju K. An update on pathogenic mechanisms of inflammatory myopathies. Curr Opin Rheumatol. 2011;23:579–84.
Nagaraju K, Lundberg IE. Polymyositis and dermatomyositis: pathophysiology. Rheum Dis Clin North Am. 2011;37:159–71. v.
Zong M, Lundberg IE. Pathogenesis, classification and treatment of inflammatory myopathies. Nat Rev Rheumatol. 2011;7:297–306.
Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci U S A. 2000;97:9209–14.
Englund P, Nennesmo I, Klareskog L, Lundberg IE. Interleukin-1alpha expression in capillaries and major histocompatibility complex class I expression in type II muscle fibers from polymyositis and dermatomyositis patients: important pathogenic features independent of inflammatory cell clusters in muscle tissue. Arthritis Rheum. 2002;46:1044–55.
Li CK, Knopp P, Moncrieffe H, Singh B, Shah S, et al. (2009) Overexpression of MHC class I heavy chain protein in young skeletal muscle leads to severe myositis. Implications for juvenile myositis. Am J Pathol.
Nogalska A, Engel WK, Askanas V. Increased BACE1 mRNA and noncoding BACE1-antisense transcript in sporadic inclusion-body myositis muscle fibers—possibly caused by endoplasmic reticulum stress. Neurosci Lett. 2010;474:140–3.
Dorn 2nd GW, Scorrano L. Two close, too close: sarcoplasmic reticulum-mitochondrial crosstalk and cardiomyocyte fate. Circ Res. 2010;107:689–99.
Ruiz-Meana M, Abellan A, Miro-Casas E, Agullo E, Garcia-Dorado D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. Am J Physiol Heart Circ Physiol. 2009;297:H1281–9.
Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–9.
Schroder JM, Molnar M. Mitochondrial abnormalities and peripheral neuropathy in inflammatory myopathy, especially inclusion body myositis. Mol Cell Biochem. 1997;174:277–81.
Miro O, Casademont J, Grau JM, Jarreta D, Urbano-Marquez A, et al. Histological and biochemical assessment of mitochondrial function in dermatomyositis. Br J Rheumatol. 1998;37:1047–53.
Alhatou MI, Sladky JT, Bagasra O, Glass JD. Mitochondrial abnormalities in dermatomyositis: characteristic pattern of neuropathology. J Mol Histol. 2004;35:615–9.
Temiz P, Weihl CC, Pestronk A. Inflammatory myopathies with mitochondrial pathology and protein aggregates. J Neurol Sci. 2009;278:25–9.
Le Fourn V, Gaplovska-Kysela K, Guhl B, Santimaria R, Zuber C, et al. Basal autophagy is involved in the degradation of the ERAD component EDEM1. Cell Mol Life Sci. 2009;66:1434–45.
Kim YS, Schwabe RF, Qian T, Lemasters JJ, Brenner DA. TRAIL-mediated apoptosis requires NF-kappaB inhibition and the mitochondrial permeability transition in human hepatoma cells. Hepatology. 2002;36:1498–508.
Mills KR, Reginato M, Debnath J, Queenan B, Brugge JS. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc Natl Acad Sci U S A. 2004;101:3438–43.
• Alger HM, Raben N, Pistilli E, Francia D, Rawat R, et al. (2011) The role of tumor necrosis factor-alpha-related apoptosis-inducing ligand (TRAIL) in mediating autophagy in myositis skeletal muscle: a potential non-immune mechanism of muscle damage. Arthritis Rheum. This article demonstrates that autophagic pathways are enhanced in the myopathic muscle and highlights the role of a nonimmune mechanism in myofiber damage in myositis.
Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum Mol Genet. 2007;16:618–29.
Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–84.
Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809.
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6.
Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–6.
Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–56.
Acknowledgments
Dr. Nagaraju is supported by a grant from the National Institutes of Health (RO1-AR050478 and AR050478-06S1). The authors wish to thank Dr. Debbie McClellan for editorial help.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rayavarapu, S., Coley, W. & Nagaraju, K. Endoplasmic Reticulum Stress in Skeletal Muscle Homeostasis and Disease. Curr Rheumatol Rep 14, 238–243 (2012). https://doi.org/10.1007/s11926-012-0247-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-012-0247-5