Abstract
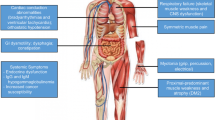
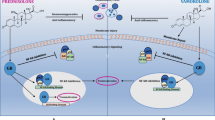
Over the past decade, the enigmatic pathogenic mechanisms of the most common forms of muscular dystrophy have been defined. In this report, the molecular defects for each of these disorders are fully described, demonstrating the potential for therapeutic intervention. In facioscapulohumeral muscular dystrophy, recent findings implicate a stabilized DUX4 transcript within the contracted D4Z4 repeats, opening the door for an RNA interference treatment strategy. In the myotonic dystrophies (dystrophica myotonia [DM]), two variants of the disease (DM1 and DM2) are caused by unrelated genes yet manifest overlapping phenotypes. The common mechanism is a splicing disorder related to RNA toxicity. Duchenne muscular dystrophy is the most common childhood form of muscular dystrophy. In many ways, the molecular gene defects are the most traditional. Gene repair strategies have advanced to the level of clinical testing, and we hope they will provide relief for this most devastating form of muscular dystrophy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Lemmers RJ, van der Vliet PJ, Klooster R. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010; 329:1650–3. This is an extremely important article that defines the molecular pathogenesis of FSHD in relation to expression of the DUX4 gene, and provides a clear path to treatment.
Wijmenga C, Frants RR, Brouwer OF, et al. Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet. 1990;336:651–3.
Tawil R, Van Der Maarel SM. Facioscapulohumeral muscular dystrophy. Muscle Nerve. 2006;34:1–15.
Ehrlich M, Jackson K, Tsumagari K, et al. Hybridization analysis of D4Z4 repeat arrays linked to FSHD. Chromosoma. 2007;116:107–16.
Tupler R, Berardinelli A, Barbierato L, et al. Monosomy of distal 4q does not cause facioscapulohumeral muscular dystrophy. J Med Genet. 1996;33:366–70.
de Greef JC, Frants RR, van der Maarel SM. Epigenetic mechanisms of facioscapulohumeral muscular dystrophy. Mutat Res. 2008;647:94–102. This is an excellent summary of the molecular defects associated with FSHD. Genetic changes are defined within the D4Z4 repeats on chromosome 4, and epigenetic modifications at the D4Z4 array are described that demonstrate that repeats induce chromatin changes that influence the appearance of the FSHD phenotype.
van Overveld PG, Lemmers RJ, Sandkuijl LA, et al. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nat Genet. 2003;35:315–7.
• Zeng W, de Greef JC, Chen YY, et al. Specific loss of histone H3 lysine 9 trimethylation and HP1gamma/cohesion binding at D4Z4 repeats in associated with facioscapulohumeral dystrophy (FSHD). PLoS Genet. 2009; 5:e10000559. This article describes biologic markers of hypomethylation that help explain the permissive environment for gene transcription of particular 4q chromosomes, which is in contrast to others that show no disease predisposition.
van der Maarel SM, Frants RR, Padberg GW. Facioscapulohumeral muscular dystrophy. Biochim Biophys Acta. 2006;1772:186–94.
Dixit M, Ansseau E, Tassin A, et al. DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proc Natl Acad Sci USA. 2007;104:18157–62.
Xia H, Mao Q, Eliason SL, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–20.
• Boudreau RL, McBride JL, Martins I, et al. Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington’s disease mice. Mol Ther. 2009; 17:1053–63. This work demonstrates the principle of RNAi therapy that can be applied to FSHD.
Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255:1253–5.
• Musova Z, Mazanec R, Krepelova A, et al. Highly unstable sequence interruptions of the CTG repeat in the myotonic dystrophy gene. Am J Med Genet. 2009; 149A:1365–74. Investigators examined the CTG repeats in DM1 patients. Pathogenic lengths were defined and the instability of intergenerational repeat lengths was described. They also identified interruptions in RNA repeats that have implications for genetic testing that can lead to false-negative conclusions.
Liquori CL, Ricker K, Moseley ML, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–7.
• Kaliman P, Llagostera E: Myotonic dystrophy protein kinase (DMPK) and its role in the pathogenesis of myotonic dystrophy 1. Cell Signal. 2008; 20:1935–41. This review attempts to better define the structural, functional, and pathophysiologic characteristics of DMPK and its relation to disease phenotype.
Margolis JM, Schoser BG, Moseley ML, et al. DM2 intronic expansions: evidence for CCUG accumulation without flanking sequence or effects on ZNF9 mRNA processing or protein expression. Hum Mol Genet. 2006;15:1808–15.
Taneja KL, McCurrach M, Schalling M, et al. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol. 1995;128:995–1002.
Miller JW, Urbinati CR, Teng-Umnuay P, et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 2000;19:4439–48.
Lin X, Miller JW, Mankodi A, et al. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genet. 2006;15:2087–97.
Kanadia RN, Johnstone KA, Mankodi A, et al. A muscleblind knockout model for myotonic dystrophy. Science. 2003;302:1978–80.
Kanadia RN, Shin J, Yuan Y, et al. Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly (CUG) model for myotonic dystrophy. Proc Natl Acad Sci USA. 2006;103:11748–53.
Kuyumcu-Martinez NM, Wang GS, Cooper TA. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell. 2007;28:68–78.
Ho TH, Bundman D, Armstong DL, et al. Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum Mol Genet. 2005;14:1539–47.
Timchenko NA, Patel R, Iakova P, et al. Overexpression of CUG triplet repeat-binding protein, CUGBP1, in mice inhibits myogenesis. J Biol Chem. 2004;279:13129–39.
• Orengo JP, Chambon P, Metzger D, et al. Expanded CTG repeats within the DMPK 3' UTR causes severe skeletal muscle wasting in an inducible mouse model for myotonic dystrophy. Proc Natl Acad Sci USA. 2008; 105:2646–51. This DM1 murine model recapitulates severe muscle wasting and shows a relation to CUGBP1 that is independent of MBLN1.
• Lee JE, Cooper TA. Pathogenic mechanisms of myotonic dystrophy. Biochem Soc Trans. 2009; 37:1281–86. This is a very well-written review of the pathogenic mechanisms involved in DM1 and DM2.
Philips AV, Timchenko LT, Cooper TA. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–41.
Savkur RS, Philips AV, Cooper TA. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet. 2001;29:40–7.
Mankodi A, Takahashi MP, Jiang H, et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell. 2002;23:3103–12.
• Raheem O, Olufemi SE, Bachinski LL, et al. Mutant (CCTG)n expansion causes abnormal expression of zinc finger protein 9 in myotonic dystrophy type 2. Am J Pathol. 2010; ePUB. This report defines a possible role for reduced ZNF9 protein as a contributory factor in DM2.
Wheeler TM et al. Correction of C1C splicing eliminates chloride channelopathy and myotonia in mouse models of myotonic dystrophy. J Clin Invest. 2007;117:3952–7.
•• Wheeler TM, Sobczak K, Lueck JD, et al. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science. 2009; 325:336–9. A morpholino AON was shown to bind to the CUG RNA repeats and block the interaction with MBNL1, thus reducing RNA toxicity. As MBLN1 was released from sequestration, the defect in alternative splicing was corrected.
• Wheeler TM. Myotonic dystrophy: therapeutic strategies for the future. Neurotherapeutics. 2008; 5:592–600. This is a very thorough review of therapeutic strategies to correct RNA toxicity in myotonic dystrophy.
•• Koshelev M, Sarma S, Price RE, et al. Heart-specific overexpression of CUGBP1 reproduces functional and molecular abnormalities of myotonic dystrophy type 1. Hum Mol Genet. 2010; 19:1066–75. The findings presented in this study demonstrate that CUGBP1 upregulation has an important role in the pathogenesis of DM1.
King WM, Ruttencutter R, Nagaraja HN, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68:1607–13.
Flanigan KM, von Niederhausern A, Dunn DM, et al. Rapid direct sequence analysis of the dystrophin gene. Am J Hum Genet. 2003;72:931–9.
Lalic T, Vossen RH, Coffa J, et al. Deletion and duplication screening in the DMD gene using MLPA. Eur J Hum Genet. 2005;13:1231–4.
Barton-Davis ER, Cordier L, Shoturma DI, et al. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest. 1999;104:375–81.
• De Luca A, Nico B, Rolland JF, et al. Gentamicin treatment in exercised mdx mice: identification of dystrophin-sensitive pathways and evaluation of efficacy in work loaded dystrophic muscle. Neurobiol Dis. 2008; 32:243–53. This study provided confirmation of an aminoglycoside effect through restoration of dystrophin in the mdx mouse challenged by increased work load through exercise.
Wagner KR, Hamed S, Hadley DW, et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol. 2001;49:706–11.
Politano L, Nigro G, Nigro V, et al. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 2003;22:15–21.
• Malik V, Rodino-Klapac LR, Viollet L, et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann Neurol. 2010; 67:771–80. This work unequivocally shows that gentamicin-induced readthrough can be achieved in a clinical setting to produce increased levels of dystrophin in boys with DMD. The clinical benefits were modest, suggesting that higher gentamicin doses or a different regimen would be necessary to produce clinically meaningful results.
Linde L, Boelz S, Nissim-Rafinia M, et al. Nonsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. J Clin Invest. 2007;117:683–92.
• Finkel RS. Read-through strategies for suppression of nonsense mutations in Duchenne/Becker muscular dystrophy: aminoglycosides and ataluren (PTC124). J Child Neurol. 2010; 25:1158–64. This article briefly reviews the strategies and progress of mutation suppression as a treatment approach for Duchenne and Becker’s muscular dystrophy.
• Lu QL, Yokota T, Takeda S, et al. The status of exon skipping as a therapeutic approach to Duchenne muscular dystrophy. Mol Ther 2010 Oct 26 [Epub ahead of print]. This article reviews the status of exon skipping for Duchenne muscular dystrophy and its potential for success as a therapeutic modality.
van Deutekom JC, Janson AA, Ginjaar IB, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–86.
•• Kinali M, Arechavala-Gomeza V, Feng L, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009; 8:918–28. This was the first successful exon skipping trial using a morpholino-based antisense oligomer to skip exon 51 following intramuscular injection of muscle. Safety and efficacy were demonstrated, and this will lead to further studies using a systemic delivery approach.
England SB, Nicholson LV, Johnson MA, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;11:180–2.
•• Mendell JR, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med. 2010; 363:1429–37. This important study shows the potential for immunogenic epitopes on revertant fibers and problems that might arise from expressing the transgene in the region of the patient’s endogenous deletion.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahenk, Z., Mendell, J.R. The Muscular Dystrophies: Distinct Pathogenic Mechanisms Invite Novel Therapeutic Approaches. Curr Rheumatol Rep 13, 199–207 (2011). https://doi.org/10.1007/s11926-011-0178-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-011-0178-6