Abstract
The rising prevalence of gout has led the pharmaceutical industry to rediscover what it had considered a forgotten disease. In April 2009, the Food and Drug Administration (FDA) approved febuxostat (Takeda Pharmaceuticals; Deerfield, IL), the first new urate-lowering gout drug in more than 40 years. In August 2009, the FDA approved colchicine for the treatment of acute gout. Several other pharmaceutical companies are also conducting clinical trials to test new drugs for acute and chronic gout. This article reviews new drugs and drugs in development in the management of acute and chronic gout.
Similar content being viewed by others
References
Papers of particular interest, published recently have been highlighted as: • Of importance •• Of major importance
Hench PS, Bauer W, Fletcher AA, et al.: Present status of the problem of “rheumatism”: review of recent American and English literature on “rheumatism” and arthritis (part 3). Ann Intern Med 1935, 8:1673–1697.
Katzung BG: Basic and Clinical Pharmacology. Edited by Katzung BG. Norwalk, CT: Appleton and Lange; 1995:536–559.
Martinon F, Petrilli V, Mayor A, et al.: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440:237–241.
• Terkeltaub B, Furst DE, Bennett K, et al.: Colchicine efficacy assessed by time to 50% reduction of pain is comparable in low dose and high dose regimens: secondary analyses of the AGREE trial. Arthritis Rheum 2009, 59:S1108. This study highlights needed information about colchicine dosing in acute gout.
Colcrys.com: Highlights of prescribing information. Available at http://www.colcrys.com/assets/pdf/COLCRYS_Full_Prescribing_Information.pdf. Accessed January 2010.
US Food and Drug Administration: Highlights of prescribing information: Colocrys. Available at www.accessdata.fda.gov/drugsatfda_docs/label/2009/022351lbl.pdf. Accessed January 2010.
Di Giovine FS, Malawista SE, Nuki G, Duff GW: Interleukin 1 (IL 1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal-induced IL 1. J Immunol 1987, 138:3213–3218.
Cronstein RN, Terkeltaub R: The inflammatory process of gout and its treatment. Arthritis Res Ther 2006, 8:S3.
•• So A, De Smedt T, Revaz S, Tschopp J: A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther 2007,12:R28. This important pilot study demonstrated that IL-1 blockade is helpful in the treatment of acute gout.
Terkeltaub R, Sundy JS, Schumacher HR, et al.: The IL-1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, nonrandomized, single-blind pilot study. Ann Rheum Dis 2009, 68:1613–1617.
•• So A, De Meulemeester M, Shamim T, et al.: Canakinumab (ACZ885) vs. triamcinolone actonide for treatment of acute gout in patients refractory or contraindicated to NSAIDs and/or colchicine. Late breaking abstract. Arthritis Rheum 2009, 60:LB4 p3660-1. This important large study in acute gout demonstrated that IL-1 blockade is helpful, especially compared with triamcinolone in the treatment of acute gout.
•• Schumacher HR, Sundy JS, Terkeltaub R, et al.: Placebo-controlled study of rilonacept for gout flare prophylaxis during initiation of urate-lowering therapy. Arthritis Rheum 2009, 59:S1096. This important study demonstrates the use of IL-1 blockade in gout prophylaxis.
Becker MA, Kisicki J, Khosravan R, et al.: Febuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteers. Nucleosides Nucleotides Nucleic Acids 2004, 23:1111–1116.
Khosravan R, Grabowski BA, Mayer MD, et al.: The effect of mild and moderate hepatic impairment on pharmacokinetics, pharmacodynamics, and safety of febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase. J Clin Pharmacol 2006, 46:88–102.
Hoshide S, Takahashi Y, Ishikawa T, et al.: PK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate kidney disease (chronic kidney disease (CKD) stages 2 and 3). Nucleosides Nucleotides Nucleic Acids 2004, 23:1117–1118. This noteworthy study demonstrated the pharmacokinetics, pharmacodynamics, and safety of febuxostat in patients with CKD 2 and 3.
Becker MA, Schumacher HR Jr, Espinosa L, et al.: A phase 3 randomized, controlled, multicenter, double-blind trial comparing efficacy and safety of daily febuxostat and allopurinol in subjects with gout. Arthritis Rheum 2008, 58:4029–4029.
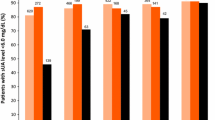
Becker MA, Schumacher HR Jr, Wortmann RL, et al.: Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005, 353:2450–2461.
Schumacher HR Jr, Becker MA, Lloyd E, et al.: Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology 2009, 48:188–194.
Varela-Echavarria A, Montes de Oca-Luna R, Barrera-Saldana HA: Uricase protein sequences: conserved during vertebrate evolution but absent in humans. The FASEB Journal 1988, 2:3092–3096.
Goldman SC, Holcenberg JS, Finklestein JZ, et al.: A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood 2001, 97:2998–3003.
Moolenburgh JD, Reinders MK, Jansen TL: Rasburicase treatment in severe tophaceous gout: a novel therapeutic option. Clin Rheumatol 2006, 25:749–752.
Richette P, Briere C, Hoenen-Clavert V, et al.: Rasburicase for tophaceous gout not treatable with allopurinol: an exploratory study. J Rheumatol 2007, 34:2093–2098.
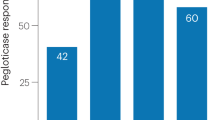
•• Sundy JS, Baraf HS, Becker MA, et al.: Efficacy and safety of intravenous (IV) pegloticase (PGL) in subjects with treatment failure gout (TFG): phase 3 results from GOUT1 and GOUT2. Arthritis Rheum 2008, 58:S635. This noteworthy study reviewed the use of pegloticase as urate-lowering therapy in patients with chronic severe gout.
Wright D, Sundy JS, Rosario-Jansen T: Routine serum uric acid (SUA) monitoring predicts antibody-mediated loss of response and infusion reaction risk during pegloticase therapy. Arthritis Rheum 2009, 59:S1104.
Maroli AN, Waltrip R, Alton M, et al.: First application of computer-assisted analysis of digital photographs for assessing tophus response: phase 3 studies of pegloticase in treatment failure gout. Arthritis Rheum 2009, 59:S1111.
Bomalaski JS, Clark MA: Serum uric acid-lowering therapies: where are we heading in management of hyperuricemia and the potential role of uricase. Curr Rheum Rep 2004, 6:240–247.
Ardea Biosciences: Development pipeline: gout. Available at http://www.ardeabio.com/gout.html. Accessed January 2010.
Disclosures
Dr. Schlesinger has received grant/research support from and served as a consultant to Novartis; served on the advisory board of Novartis, Takeda, Savient, URL, Pharma, and EnzymeRx; and served on the speakers’ bureau of Takeda.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlesinger, N. New Agents for the Treatment of Gout and Hyperuricemia: Febuxostat, Puricase, and Beyond. Curr Rheumatol Rep 12, 130–134 (2010). https://doi.org/10.1007/s11926-010-0093-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-010-0093-2