Abstract
Purpose of Review
To consider various precision medicine approaches to further elucidate the relationship between inflammation and depression and to illustrate how a neurodevelopmental perspective can help in this regard.
Recent Findings
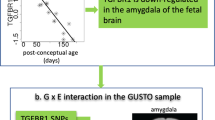
Inflammation associates most strongly with phenotypes of depression that reflect illness behavior and/or metabolic dysfunction and obesity. A separate body of research has shown that maternal inflammation during pregnancy can alter brain circuitry important for mood regulation and/or reward in the developing fetus. Our research group is finding that maternal CRP levels differentially predict positive and negative affect in children assessed at age 4 years, depending on the timing of plasma sampling during pregnancy and the sex of the child.
Summary
Recent authors have stressed the need to use a variety of precision medicine approaches to refine our understanding of inflammation—depression links. Adding a neurodevelopmental perspective may help to address some of the methodological challenges in this active area of study.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Felger JC, Miller AH. Identifying immunophenotypes of inflammation in depression: dismantling the monolith. Biol Psychiatry. 2020;88(2):136–8. This review highlights the importance of using a precision medicine approach to improve our understanding of depression-inflammation links.
Lee Y, Subramaniapillai M, Brietzke E, Mansur RB, Ho RC, Yim SJ, et al. Anti-cytokine agents for anhedonia: targeting inflammation and the immune system to treat dimensional disturbances in depression. Ther Adv Psychopharmacol. 2018;8(12):337–48.
Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30(1):1–16.
Cuthbert BN. Translating intermediate phenotypes to psychopathology: the NIMH research domain criteria. Psychophysiology. 2014;51(12):1205–6.
• Majd M, Saunders EFH, Engeland CG. Inflammation and the dimensions of depression: a review. Front Neuroendocrinol. 2020;56:100800. This review demonstrates which symptoms of depression are most strongly associated with inflammation.
Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alpha-2b therapy. J Clin Oncol. 2000;18:2143–51.
Dantzer R. Cytokine-induced sickness behavior. Brain Behav Immun. 2001;17:222–34.
Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: anhedonia and neurochemical changes. Brain Behav Immun. 2002;16:544–56.
van Eeden WA, van Hemert AM, Carlier IVE, Penninx BWJH, Lamers F, Fried EI, et al. Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression. Transl Psychiatry. 2020;10(1):235.
Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, Bower JE. The role of inflammation in core features of depression: insights from paradigms using exogenously-induced inflammation. Neurosci Biobehav Rev. 2018;94:219–37.
• Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, et al. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry. 2018;8(1):189. This paper demonstrates how imaging can help us identify inflammatory phenotypes of depression.
•• Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21(10):1358–65. This paper shows how inflammation can alter brain reward pathways in depression. The early developmental data that we present in the current manuscript extend particularly well from this finding.
Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS. Mapping inflammation onto mood: inflammatory mediators of anhedonia. Neurosci Biobehav Rev. 2016;64:148–66.
Treadway MT, Admon R, Arulpragasam AR, Mehta M, Douglas S, Vitaliano G, et al. Association between Interleukin-6 and striatal prediction-error signals following acute stress in healthy female participants. Biol Psychiatry. 2017;82(8):570–7.
• Moieni M, Tan KM, Inagaki TK, Muscatell KA, Dutcher JM, Jevtic I, et al. Sex Differences in the relationship between inflammation and reward sensitivity: a randomized controlled trial of endotoxin. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(7):619–26. The preliminary sex differences data we present in the current manuscript are highly consistent with this finding.
Bradley KA, Stern ER, Alonso CM, Xie H, Kim-Schulze S, Gabbay V. Relationships between neural activation during a reward task and peripheral cytokine levels in youth with diverse psychiatric symptoms. Brain Behav Immun. 2019;80:374–83.
Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18(6):692–9.
•• Lamers F, Milaneschi Y, Vinkers CH, Schoevers RA, Giltay EJ, Penninx BWJH. Depression profilers and immuno-metabolic dysregulation: Longitudinal results from the NESDA study. Brain Behav Immun. 2020;88:174–83. This paper addresses many of the methodological issues in this area of work and how they might be resolved. The large sample and longitudinal aspect of the data are also very informative.
Hickman RJ, Khambaty T, Stewart JC. C-reactive protein is elevated in atypical but not nonatypical depression: data from the National Health and Nutrition Examination survey (NHANES) 1999-2004. J Behav Med. 2014;37(4):621–9.
Glaus J, von Känel R, Lasserre AM, Strippoli MF, Van Deleur CL, Castelao E, et al. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol Med. 2018;48(6):961–73.
Yang C, Tiemessen KM, Bosker FJ, Wardenaar KJ, Lie J, Schoevers RA. Interleukin, tumor necrosis factor-α and C-reactive protein profiles in melancholic and non-melancholic depression: a systematic review. J Psychosom Res. 2018;111:58–68.
Tsuriya D, Morita H, Morioka T, Takahashi N, Ito T, Oki Y, et al. Significant correlation between visceral adiposity and high-sensitivity C-reactive protein (hs-CRP) in Japanese subjects. Intern Med. 2011;50(22):2767–73.
Sanip Z, Ariffin FD, Al-Tahami BA, Sulaiman WA, Rasool AH. Obesity indices and metabolic markers are related to hs-CRP and adiponectin levels in overweight and obese females. Obes Res Clin Pract. 2013;7(4):e315–20.
• Woelfer M, Kasties V, Kahlfuss S, Walter M. The role of depressive subtypes within the neuroinflammation hypothesis of major depressive disorder. Neuroscience. 2019;403:93–110. This extensive review uses a precision medicine approach to identify and critically appraise various clinical phenotypes of depression tied to inflammation.
Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353(6301):772–7.
Baines KJ, Hillier DM, Haddad FL, Rajakumar N, Schmid S, Renaud SJ. Maternal immune activation alters fetal brain development and enhances proliferation of neural precursor cells in rats. Front Immunol. 2020;11:1145.
Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014;171(9):960–8.
van der Burg JW, Sen S, Chomitz VR, Seidell JC, Leviton A, Dammann O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr Res. 2016;79(1–1):3–12.
Azhari A, Azizan F, Esposito G. A systematic review of gut-immune-brain mechanisms in Autism Spectrum Disorder. Dev Psychobiol. 2019 Jul;61(5):752–71.
• Gumusoglu SB, Stevens HE. Maternal inflammation and neurodevelopmental programming: a review of preclinical outcomes and implications for translational psychiatry. Biol Psychiatry. 2019;85(2):107–21. This paper describes how maternal immune changes might lead to altered fetal brain development and psychiatric disorders.
Patel S, Cooper MN, Jones H, Whitehouse AJO, Dale RC, Guastella AJ. Maternal immune-related conditions during pregnancy may be a risk factor for neuropsychiatric problems in offspring throughout childhood and adolescence. Psychol Med. 2020;1:1–11.
Stewart JW, Bruder GE, McGrath PJ, Quitkin FM. Do age of onset and course of illness define biologically distinct groups within atypical depression? J Abnorm Psychol. 2003;112(2):253–62.
Gustafsson HC, Sullivan EL, Nousen EK, Sullivan CA, Huang E, Rincon M, et al. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain Behav Immun. 2018;73:470–81.
•• Mac Giollabhui N, Breen EC, Murphy SK, Maxwell SD, Cohn BA, Krigbaum NY, et al. Maternal inflammation during pregnancy and offspring psychiatric symptoms in childhood: timing and sex matter. J Psychiatr Res. 2019;111:96–103. This paper identifies two key moderators for neurodevelopmental work based on maternal inflammation in pregnancy. These proved very helpful in interpreting the current data and demonstrating how precision medicine might apply to neurodevelopmental work in general.
Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci. 2018;21(5):765–72.
Anderson LN, Knight JA, Hung RJ, Hewko SL, Seeto RA, Martin MJ, et al. The Ontario Birth Study: a prospective pregnancy cohort study integrating perinatal research into clinical care. Paediatr Perinat Epidemiol. 2018;32(3):290–301.
Straley ME, Van Oeffelen W, Theze S, Sullivan AM, O'Mahony SM, Cryan JF, et al. Distinct alterations in motor & reward seeking behavior are dependent on the gestational age of exposure to LPS-induced maternal immune activation. Brain Behav Immun. 2017;63:21–34.
Marín O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med. 2016;22(11):1229–38. https://doi.org/10.1038/nm.4225.
Davis EP, Pfaff D. Sexually dimorphic responses to early adversity:implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. 2014;49:11–25.
Alves JM, Luo S, Chow T, Herting M, Xiang AH, Page KA. Sex differences in the association between prenatal exposure to maternal obesity and hippocampal volume in children. Brain Behav. 2020;10(2):e01522.
Hicks LM, Swales DA, Garcia SE, Driver C, Davis EP. Does prenatal maternal distress contribute to sex differences in child psychopathology? Curr Psychiatry Rep. 2019;21(2):7.
Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, et al. Emotion assessment using the NIH toolbox. Neurology. 2013;80(11 Suppl 3):S76–86.
Acknowledgments
The authors acknowledge the contribution and support of the Ontario Birth Study Team members. In addition, we thank and are extremely grateful to all the women who took part in this study.
Funding
Funding for this project was provided by the Cameron Parker Holcombe Wilson Chair in Depression Studies at CAMH and University of Toronto (awarded to Dr. Levitan), and the Alva Foundation. Funding for the Ontario Birth Study has been provided by Mount Sinai Hospital, Mount Sinai Hospital Foundation, and the Lunenfeld-Tanenbaum Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The various components of this study including the informed consent protocol for the OBS and OBS-KIDS were approved by the research ethics boards of Mt. Sinai Hospital, the University of Toronto and CAMH.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Precision Medicine in Psychiatry
Rights and permissions
About this article
Cite this article
Levitan, R.D., Zhang, C.X.W., Knight, J.A. et al. Using Precision Medicine with a Neurodevelopmental Perspective to Study Inflammation and Depression. Curr Psychiatry Rep 22, 87 (2020). https://doi.org/10.1007/s11920-020-01206-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11920-020-01206-8