Abstract
Purpose of Review
The purpose of this review is to provide a background of osteoporosis and air pollution, discussing increasing incidence of the disease with exposure to pollutants and the role that inflammation may play in this process.
Recent Findings
Osteoporosis-related fractures are one of the most pressing challenges for the ageing global population, with significant increases in mortality known to occur after major osteoporotic fractures in the elderly population. Recent studies have established a firm correlative link between areas of high air pollution and increased risk of osteoporosis, particularly alarming given the increasingly urban global population. While the culprit pollutants and molecular mechanisms underlying this phenomenon have not yet been elucidated, initial studies suggest a role for inflammatory cascades in this phenomenon.
Summary
While much more research is required to identify the most damaging air pollutants and to delineate the specific inflammatory molecular mechanisms, it is clear from the literature that shedding light on these pathways would unveil potential therapeutic targets to treat bone diseases, including osteoporosis. Major deficiencies of current animal models highlight the need for complex human in vitro models such as organ-on-a-chip technology to better understand the impact of air pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This review begins with a brief discussion of the the key drivers of osteoporosis, and the current standard of care. This is then followed by a review of air pollutants, their influence on human disease and the role played by inflammation in these conditions. The article next explores the putative link between air pollution and osteoporosis, discussing the state-of-the-art in the field, before concluding with a future perspective on the potential of targeting air pollution and related inflammatory pathways to inhibit the development of osteoporosis.
Osteoporosis
Osteoporosis presents as loss of bone mass, leading to fractures, severe pain, deformity and increased rates of mortality [1]. Clinically, the disease is classified as either primary or secondary osteoporosis. Primary osteoporosis refers to both bone loss occurring due to oestrogen deficiency in post-menopausal women (type I) and bone loss associated with the normal ageing process (type II). Secondary osteoporosis describes bone loss that occurs due to other diseases (e.g. cancer) or drug treatment (e.g. chemotherapies). Post-menopausal osteoporosis (type I), as the most common diagnosis, arises as the result of deficient oestrogen following the menopause [2].
Healthy bone maintains its strength and mineral homeostasis via bone remodelling, which is a coordinated and balanced process whereby osteoclasts continuously resorb aged or damaged bone and osteoblasts reform new bone tissue in its place [3]. However, this balance is perturbed during oestrogen deficiency, with osteoclasts removing excess bone without adequate formation by osteoblasts [4]. With the continuation of this process, bone loss manifests when trabeculae (internal supporting struts of bone) become thin and resorb completely, or fracture [5]. Eventually, this process allows debilitating bone fractures to occur under minimal trauma in the bones of the hip, wrist and spine.
The healthy remodelling process is also disrupted by disuse due to skeletal mechanical unloading [6]. A range of mechanosensing mechanisms exist in bone cells, such as mesenchymal stromal cells (MSCs) [7], including the primary cilium, a solitary sensory organelle that protrudes from the membrane of all bone cells that has been shown to act as key mediators of inflammatory signalling and mechanotransduction [8]. Mechanical stimulation via primary cilia, for instance by oscillatory fluid flow-induced shear stress, triggers osteogenic differentiation [9]. Primary cilium expression is similarly crucial in the process of osteoclastogenisis, with recent work demonstrating that increased primary cilium expression can inhibit osteoclast formation [10]. Furthermore, the primary cilium is well known to play an important role in mechanotransduction by osteocytes [11,12,13], thought to be the master orchestrator of bone adaptation to mechanical loading in health [14] and during osteoporosis [15, 16]. Thus, lack of mechanical stimulation can ultimately lead to imbalance of bone remodelling.
Age-related fractures are increasingly common. For example, in the US approximately ∼2.1 million osteoporosis-related bone fractures occur annually [17, 18]. Osteoporosis impacts women more than men, with 80% of the estimated 10 million Americans with osteoporosis being women. and one in two women over 50 experiencing a bone fracture because of osteoporosis [19]. Indeed, women over 45 years of age spend more days in hospital due to osteoporosis than diabetes, heart attack or breast cancer [20].
While a number of established diagnosis and treatment options exist for osteoporosis, clear deficiencies remain, highlighting the need for further research into treatment and prevention. Indeed, with a rapidly growing global population of ageing individuals, uncovering new mechanisms underlying the development of osteoporosis and ways to mitigate them is becoming increasingly urgent. Even more concerning, given the increasingly urban world population, air pollutants have recently been implicated in the development of osteoporosis, as will be discussed hereafter.
Air Pollution
Air pollution has been highlighted as the greatest environmental threat to individual human health; according to the World Health Organization (WHO), 99% of the population breathe air that exceeds their guidelines on safe pollutant levels [21]. A 2019 study suggested that excessive levels of air pollution may be responsible for 8.79 million deaths per year globally [22]. Exposure to high levels of air pollution increases the risk of developing numerous diseases with significant effects on morbidity and mortality. Diseases currently linked to air pollution include cardiovascular disease [22], cancer [23], respiratory diseases [24], diabetes mellitus [25], immune disorders [26], and neurological disorders [27]. It is estimated that worldwide, each year seven million deaths can be attributed to the effects of ambient and household air pollution [21].
Air pollution is generally defined as solid, liquid and gaseous compounds that affect biological systems through one mechanism or another. Major sources of air pollution include vehicle emissions, industrial processes, power generation, and wildfires. Forms of air pollution can include gases such as ozone (O3), and noxious gases such as carbon dioxide (CO2), carbon monoxide (CO), nitrogen oxides (NO, NO2) and sulphur oxides (SO, SO2), as well as volatile organic compounds [28]. Pollution can also include particulate matter (PM), which can be classified according to the nature of particles, as biological, chemical, mineral and metal. However, while varied in nature, their inflammatory action is classified based on particle size, with diameter of PM ≤ 10 μm, ≤ 2.5 μm, ≤ 1 μm, ≤ 100 nm all classified as coarse particles (PM10), and in order of decreasing size fine particles (PM2.5), very fine particles (PM1.0) and ultrafine particles (PM0.1 or UFPs), respectively. Although the mechanism of air pollution affecting the lungs is obvious, how air pollutants can affect other body systems is still poorly understood and an area of broad study.
Air Pollutants and Inflammation
The effects of air pollution on organs distant from the lungs, the site of inhalation, is thought to lead to health defects due to oxidative stress or inflammation [29]. While it is currently unclear which components of air pollution may trigger immune and inflammatory responses, and by what mechanism, there are multiple studies into the various types of pollutants and the diseases they are linked to.
Particulate matter, comprising extremely small particles, is able to enter the bloodstream via inhalation, and is known to trigger the systemic release of proinflammatory cytokines, including TNF-α, IL-1, IL-6 and IL-8 [26, 30, 31], and to elevate the incidence and severity of autoimmune disease [32]. Increased levels of these cytokines in systemic circulation may lead to an increase activity of immune cells and induce tissue damage.
PM2.5 exposure has been associated with elevated levels of circulating monocytes and T cells, but not B cells [33], suggesting activation of T cells via receptors or pathways specific to these immune cells. This is further supported by a study that found that polluted air caused an imbalance of T cells, leading to increased production of proinflammatory cytokines, oxidative stress, and methylation changes [26]. An alternative proposed mechanism of action is that air pollution leads to damaged mitochondria, triggering oxidative stress, which causes an over-production of inflammatory cytokines, and the stimulation of T helper lymphocytes type 1 (Th1) production [26].
Long term exposure to PM2.5 leading to increased cytokine expression has been associated with cardiovascular disease [31], as well as increased incidence of Alzheimer’s disease [34]. Furthermore, in vitro and in vivo studies have found that PM induces high levels of several inflammatory markers, including IL-1a, IL-1B, IL-6, IL-8, IL-17, and TNF-α, in the lungs [35, 36]. Another air pollution study linked elevated exposure to NO2 to increased systemic inflammation in COPD patients [37]. Thus, when individuals are exposed to air pollution, there are likely multiple pollutants triggering a range of immune responses simultaneously, activating a variety of pathways that lead to the development of a particular disease. Given this complexity, specific molecular mechanisms are difficult to target clinically, and both fundamental science and drug discovery in this space will rely on improvements in in vivo and in vitro models of these diseases.
Linking Air Pollutants and Bone Health
A number of studies (outlined in Table 1), with increasing pace in the last five years, have shown that in addition to affecting many other physiological systems, a strong link exists between air pollution and bone degeneration. Early indications of a potential relationship between air pollution and bone health arose a 2007 study of Norwegian populations, with an Oslo-based study finding a weak, but still significant, correlation that air pollution was inversely associated with total body BMD [38]. Two additional studies found in 2010 that increased levels of outdoor air pollution could be correlated with loss of bone density and increased rates of forearm fracture [39], and in 2011 that urban women have a 29% higher relative risk of forearm fracture and reduced bone mineral density compared to women in rural areas [40], further hinting that air pollution could affect bone health. Later, in 2015 researchers found similar results in Mexican American populations, reporting a relationship between road traffic metrics, associated ambient air pollution and low BMD [41]. Despite these findings, a systematic review in 2021 found that the links between particulate pollution and osteoporosis are inconclusive, partly due to heterogeneity in study design and subject populations [42].
It has been shown that short-term air pollution exposure increases hip fracture risk in multiple European populations (Fig. 1A) [44, 48]. Similar associations have also been found in multiple human studies across a wide range of countries in Asia [46, 47, 56,57,58].
A Long-term exposure to PM10 in Italy (2013–2019 average concentration μg/m3)[48]. Risk of osteoporosis at any site in patients chronically exposed to PM10 > 30 μg/m3 and PM2.5 > 25 μg/m3. Model 1 adjusted for age, body mass index (BMI), presence of prevalent fragility fractures, family history of osteoporosis, and menopause. Model 2 adjusted for age, BMI, presence of prevalent fragility fractures, family history of osteoporosis, menopause, glucocorticoid treatment, and comorbidities. Model 3 (main model) adjusted for age, BMI, presence of prevalent fragility fractures, family history of osteoporosis, menopause, glucocorticoid treatment, comorbidities, and macro-area of residency (categorized as northern Italy, central Italy, and southern Italy). B Average PM2.5 concentrations per zipcode in the US Northeast between 2003 and 2010[43]. Spline shown for the multivariable-adjusted association between PM2·5 exposure and number of hospital admissions of Medicare enrollees per zipcode, from 2003 to 2010. Horizontal dotted line represents zero effect. C Example of nearest neighbour interpolation between measurements in Taiwan [58], with big circles standing for monitoring station and small ones for participants. A synergistic effect of CO and NOx on BMD T-score was found to be statistically significant (p = 0.001), as was a synergistic effect between SO2 and NO2 (p = 0.004)
Perhaps most importantly, a recent landmark paper has prospectively determined the impact of criteria air pollutants and their mixtures on BMD in ~ 161,000 postmenopausal women in the US [49], using two separate epidemiological studies to reveal a correlation between air pollution and a ninefold increase in risk of osteoporosis (Fig. 1B) and with general bone damage [43]. This study demonstrated for the first time that from air pollution mixtures, nitrogen oxides likely contribute the most to bone damage and that the lumbar spine is one of the most susceptible sites [43]. Results from these analyses indicated that poor air quality was a possible risk factor for BMD loss and fractures in older individuals and that per each 4.18 μg/m3 increase in PM2.5, there is a 4.1% higher rate of hospital admission for bone fractures in older individuals [43, 49]. Thus, in studies using very large population sizes, there now appears to be a clear and significant link between air pollutants and bone health, but the potential underlying mechanism is as yet undiscovered.
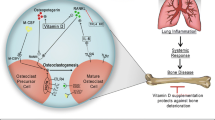
This Lancet study by Prada et al. [43] was quickly followed by a flurry of epidemiological studies demonstrating the same effect in other countries [59], including a number leveraging the unique dataset held within the UK Biobank [50,51,52,53]. For example, recent reports from Zhang et al. suggested that long-term exposure to PM2.5 was associated with decreased BMD T-score and increased osteoporosis risk among participants from rural areas of China [54]. The UK Biobank studies in particular found clear links between a range of air pollutants and decreased bone mass, decreased BMD and increased risk of fracture within the UK population [50,51,52], particularly identifying PM2.5 and nitrogen oxides as likely culprit pollutants. A recent additional study applied Mendelian randomisation on UK biobank data, which employed statistical analysis to develop greater confidence in causal links between variables, finding robust stastical evidence affirming a causal relationship between decrease in BMD and increased PM2.5, PM10, NO and NO2 exposure [55]. A number of putative mechanisms have been proposed, all of which generally involve inflammatory signalling [60, 61]; 1) low-grade systemic inflammation affecting osteoblast and osteoclast differentiation and function; 2) oxidative damage in the airway and bone cells from compounds such as heavy metals; 3) endocrine disruption when binding to the receptors in bone cell; and 4) directly or indirectly inducing vitamin D deficiency. However, at present, the specific inflammatory mechanism that causes osteoporosis remains unknown.
Inflammation influences various important signalling pathways in bone health; the release of pro-inflammatory cytokines has been reported to inhibit osteoblast mitogen-activated protein kinases (MAPK) [62] and the WNT–Frizzled–β-catenin pathway [63, 64] that ultimately suppresses the differentiation and activation of osteoblasts. In osteoclasts, activation via inflammatory mechanisms have been shown to amplify osteoclastogenesis, resulting in local bone loss [65].
Previous research into the effect of inflammation on primary cilia showed that cilium length was elongated following IL-1β exposure [66]. Primary cilia mediate a number of key inflammatory pathways in osteocytes [67], and have been shown to play a role in downstream inflammatory signalling [68], increasing the release of inflammatory mediators within bone, and potentially altering the cells’ functional mechanosensation. Similarly, in the context of breast and bone cancer, the osteocyte primary cilium has been shown to mediate TGF-β and TNF-α inflammatory signalling in the metastatic niche [69], highlighting this organelle as a potential target for air-pollution mediated inflammation.
Air pollution-induced osteoporosis is therefore a significant challenge for health systems, as the global population is rapidly ageing and mortality increases substantially in elderly patients in the years after a hip or vertebral fracture. Most importantly, the global population is increasingly urban and exposed to these pollutants, with the UN predicting 68% of the global population residing in cities by 2050 [70]. Demonstrating the importance of place, specific localities and social groups are exposed to poorer air quality and therefore higher risk of bone degeneration.
A key challenge to identifying the molecular mechanism underlying these destructive relationships, as demonstrated by the few animal studies on the topic [45, 53], is that rodent models do not age or remodel bone in the same manner as humans, and do not naturally develop osteoporosis. This is especially true given that the mechanisms likely involve lung-immune-bone crosstalk, and rodents have been shown to have vastly different immune and healing responses to humans [71]. Indeed, the first animal study carried out found contradictory interactions, with little indication of bone damage in a rat model resulting from air pollutants and increased blood levels of vitamin D due to exposure to some air pollutants [45]. The only other animal study to date, performed on male C57BL/6 mice, did indeed find that PM2.5 exposure resulted in increased osteoclastogenesis, dysregulated osteogenesis and shortened femur length, although no significant differences in femur structure or BMD were detected [53]. This study did also conduct a simple in vitro experiment, in which they found that osteoclastogenic behaviour and signalling was disrupted by conditioned media from macrophages exposed to PM2.5 [53]. Taken together, these limited experiments suggest that further investigation to unpick these molecular mechanisms is likely to require sufficiently complex human-derived in vitro models that can include components of the immune system (e.g. organ-on-a-chip or microphysiological systems) [72, 73]. Indeed, guidance from regulatory agencies (e.g. FDA, EMA) and funding bodies (e.g. NIH, Horizon Europe) worldwide has been updated in the past five years to encourage the development of more accurate in vitro models, including to address conditions with complex immune involvement as may occur in pollution-related skeletal degeneration.
Considering the expanding body of evidence implicating the effects of air pollution on various organ systems, paired with the research into inflammation leading to loss of BMD and increased fracture risks, it logically follows that air pollution triggers an inflammatory response in bones, leading to degeneration and diseases like osteoporosis. As there has been little research to study the effect air pollution has on bone health, the precise mechanisms are currently unknown.
Conclusions
Research over the past five years has established a link between air pollution and bone degeneration, and an association with an increased fracture risk. Public interest in this challenge recently highlighted in an article in Science [74]. Increased risk of osteoporosis has been specifically identified, implying that systemic inflammatory factors may induce early onset of osteoporosis. Mounting evidence appears to identify nitrogen oxides and PM2.5 as irritants of key interest. However, while major steps have been taken in understanding the epidemiological and population-level associations, the precise mechanisms through which these pollutants induce bone damage or instigate osteoporotic cascades remain to be elucidated. Further study is required to identify the impact of different types of pollutants, the resulting impact of inflammation on bone health and the underlying biological pathways. Given the deficiencies of animal models of air pollution and bone diseases, it is clear that new complex human in vitro models such as organ-on-a-chip technology will be required in this field.
Key References
-
Prada, D.; Zhong, J.; Colicino, E.; Zanobetti, A.; Schwartz, J.; Dagincourt, N.; Fang, S.C.; Kloog, I.; Zmuda, J.M.; Holick, M. Association of air particulate pollution with bone loss over time and bone fracture risk: analysis of data from two independent studies. Lancet Planet. Heal. 2017, 1, e337–e347.
-
This paper used extremely large datasets in multiple cohorts to establish strong statistical evidence that poor air quality is a modifiable risk factor for bone fractures and osteoporosis, especially in low-income communities.
-
-
Kheirouri, S.; Alizadeh, M.; Abad, R.M.S.; Barkabi-Zanjani, S.; Mesgari-Abbasi, M. Effects of sulfur dioxide, ozone, and ambient air pollution on bone metabolism related biochemical parameters in a rat model. Environ. Anal. Heal. Toxicol. 2020, 35.
-
This study represents the first in vivo experiment to investigate the link between air pollutants and bone health, with contradicting findings suggesting that more complex in vitro models are required to establish an underpinning mechanism.
-
-
Ge Q, Yang S, Qian Y, Chen J, Yuan W, Li S, Wang P, Li R, Zhang L, Chen G, Kan H. Ambient PM 2.5 Exposure and Bone Homeostasis: Analysis of UK Biobank Data and Experimental Studies in Mice and in Vitro. Environmental Health Perspectives. 2023 Oct 4;131(10):107002.
-
This study conducts both in vivo mouse and in vitro conditioned media experiment to investigate the effects of PM2.5 pollutants, finding disruption to osteoclastogenisis and osteoclastic signalling in both models. However, neither finds strong indications of loss in bone mineral, further suggesting that more complex in vitro models are required.
-
-
Yu, X.-H.; Cao, H.-W.; Bo, L.; Lei, S.-F.; Deng, F.-Y. Air pollution, genetic factors and the risk of osteoporosis: A prospective study in the UK biobank. Front. Public Heal. 2023, 11, 1119774.
-
This study applied the resources of the UK Biobank to find that that exposure to various air pollutants, individually or jointly, could improve the risk of developing OP and fractures, and increased the risk by interacting with genetic factors.
-
-
Jiang R, Qu Q, Wang Z, Luo F, Mou S. Association between air pollution and bone mineral density: a Mendelian randomization study. Archives of Medical Science. 2024.
-
This study further leveraged the data held within the UK biobank, applying a Mendelian randomisation method to give statistical confidence of a robust causal link between lower BMD and exposure to nitrogen oxides and particulate matter.
-
Data Availability
Data presented and discussed in this review is available at source in the relevant referenced studies.
References
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jönsson B. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15:38–42.
Riggs BL, Khosla S, Melton LJ III. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302.
Parfitt AM. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int. 1984;36:S37–45.
McNamara LM. Perspective on post-menopausal osteoporosis: establishing an interdisciplinary understanding of the sequence of events from the molecular level to whole bone fractures. J R Soc Interface. 2010;7:353–72.
Eriksen EF, Hodgson SF, Eastell R, RIGGS BL, Cedel SL, O’Fallon WM. Cancellous bone remodeling in type I (postmenopausal) osteoporosis: quantitative assessment of rates of formation, resorption, and bone loss at tissue and cellular levels. J Bone Miner Res 1990, 5, 311–319.
Alexandre C, Vico L. Pathophysiology of bone loss in disuse osteoporosis. Jt Bone Spine. 2011;78:572–6. https://doi.org/10.1016/j.jbspin.2011.04.007.
Verbruggen SW, McNamara LM. Chapter 6 - Bone mechanobiology in health and disease. In: Verbruggen WS, editor. Mechanobiology in health and disease. Academic Press; 2018. pp. 157–214. https://doi.org/10.1016/B978-0-12-812952-4.00006-4.
Hoey DA, Tormey S, Ramcharan S, O’Brien FJ, Jacobs CR. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells. 2012;30:2561–70. https://doi.org/10.1002/stem.1235.
Kelly DJ, Jacobs CR. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res Part C Embryo Today Rev. 2010;90:75–85.
Sutton MM, Duffy MP, Verbruggen SW, Jacobs CR. Osteoclastogenesis requires primary cilia disassembly and can be inhibited by promoting primary cilia formation pharmacologically. Cells Tissues Organs. 2024;213:235–44.
Vaughan TJ, Mullen CA, Verbruggen SW, McNamara LM. Bone cell mechanosensation of fluid flow stimulation: a fluid–structure interaction model characterising the role integrin attachments and primary cilia. Biomech Model Mechanobiol. 2015;14:703–18. https://doi.org/10.1007/s10237-014-0631-3.
Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun. 2011;412:182–7. https://doi.org/10.1016/j.bbrc.2011.07.072.
Malone AMD, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–30. https://doi.org/10.1073/pnas.0700636104.
Schaffler MB, Cheung W-Y, Majeska R, Kennedy O. Osteocytes: Master orchestrators of bone. Calcif Tissue Int. 2014;94:5–24. https://doi.org/10.1007/s00223-013-9790-y.
Verbruggen SW, Vaughan TJ, McNamara LM. Mechanisms of osteocyte stimulation in osteoporosis. J Mech Behav Biomed Mater. 2016. https://doi.org/10.1016/j.jmbbm.2016.05.004.
Verbruggen SW, Mc Garrigle MJ, Haugh MG, Voisin MC, McNamara LM. Altered mechanical environment of bone cells in an animal model of short- and long-term osteoporosis. Biophys J. 2015;108:1587–98. https://doi.org/10.1016/j.bpj.2015.02.031.
Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. Br Med Bull. 2020;133:105–17.
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J bone Miner Res. 2007;22:465–75.
Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22:671–85.
Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D. obot Guidelines for diagnosis and management of osteoporosis. Osteoporos Int. 1997;7:390–406.
World Health Organization. WHO global air quality guidelines: particulate matter (PM2. 5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. World Health Organization; 2021.
Lelieveld J, Klingmüller K, Pozzer A, Pöschl U, Fnais M, Daiber A, Münzel T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. 2019;40:1590–6.
Guo H, Chang Z, Wu J, Li W. Air pollution and lung cancer incidence in China: Who are faced with a greater effect? Environ Int. 2019;132:105077.
Luo L, Zhang Y, Jiang J, Luan H, Yu C, Nan P, Luo B, You M. Short-term effects of ambient air pollution on hospitalization for respiratory disease in Taiyuan, China: a time-series analysis. Int J Environ Res Public Health. 2018;15:2160.
Yang B-Y, Fan S, Thiering E, Seissler J, Nowak D, Dong G-H, Heinrich J. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res. 2020;180:108817.
Zhao C-N, Xu Z, Wu G-C, Mao Y-M, Liu L-N, Dan Y-L, Tao S-S, Zhang Q, Sam NB, Fan Y-G. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev. 2019;18:607–14.
Schikowski T, Altuğ H. The role of air pollution in cognitive impairment and decline. Neurochem Int. 2020;136:104708.
Miller MR. Oxidative stress and the cardiovascular effects of air pollution. Free Radic Biol Med. 2020;151:69–87.
Rückerl R, Schneider A, Breitner S, Cyrys J, Peters A. Health effects of particulate air pollution: a review of epidemiological evidence. Inhal Toxicol. 2011;23:555–92.
Steenhof M, Gosens I, Strak M, Godri KJ, Hoek G, Cassee FR, Mudway IS, Kelly FJ, Harrison RM, Lebret E. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential-the RAPTES project. Part Fibre Toxicol. 2011;8:1–15.
Chen R, Li H, Cai J, Wang C, Lin Z, Liu C, Niu Y, Zhao Z, Li W, Kan H. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ Health Perspect. 2018;126:17007.
Gawda A, Majka G, Nowak B, Marcinkiewicz J. Air pollution, oxidative stress, and exacerbation of autoimmune diseases. Cent Eur J Immunol. 2017;42:305–12.
Pope CA III, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119:1204–14.
Jung C-R, Lin Y-T, Hwang B-F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimer’s Dis. 2015;44:573–84.
Øvrevik J, Låg M, Holme JA, Schwarze PE, Refsnes M. Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology. 2009;259:46–53. https://doi.org/10.1016/j.tox.2009.01.028.
Farina F, Sancini G, Battaglia C, Tinaglia V, Mantecca P, Camatini M, Palestini P. Milano summer particulate matter (PM10) triggers lung inflammation and extra pulmonary adverse events in mice. PLoS ONE. 2013;8:e56636.
Dadvand P, Nieuwenhuijsen MJ, Agustí À, De Batlle J, Benet M, Beelen R, Cirach M, Martinez D, Hoek G, Basagaña X, Ferrer A. Air pollution and biomarkers of systemic inflammation and tissue repair in COPD patients. European Respiratory Journal. 2014;44(3):603–13. https://doi.org/10.1183/09031936.00168813.
Alvaer K, Meyer HE, Falch JA, Nafstad P, Søgaard AJ. Outdoor air pollution and bone mineral density in elderly men-the Oslo Health Study. Osteoporos Int. 2007;18:1669–74.
Alver K, Meyer HE, Falch JA, Søgaard AJ. Outdoor air pollution, bone density and self-reported forearm fracture: the Oslo Health Study. Osteoporos Int. 2010;21:1751–60.
Omsland TK, Ahmed LA, Grønskag A, Schei B, Emaus N, Langhammer A, Joakimsen RM, Jørgensen L, Søgaard AJ, Gjesdal CG. More forearm fractures among urban than rural women: the NOREPOS study based on the Tromsø study and the HUNT study. J bone Miner Res. 2011;26:850–6.
Chen Z, Salam MT, Karim R, Toledo-Corral CM, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F. Living near a freeway is associated with lower bone mineral density among Mexican Americans. Osteoporos Int. 2015;26:1713–21.
Pang KL, Ekeuku SO, Chin KY. Particulate air pollution and osteoporosis: a systematic review. Risk Manag Healthcare Policy. 2021;14:2715–32.
Prada D, Zhong J, Colicino E, Zanobetti A, Schwartz J, Dagincourt N, Fang SC, Kloog I, Zmuda JM, Holick M. Association of air particulate pollution with bone loss over time and bone fracture risk: analysis of data from two independent studies. Lancet Planet Heal. 2017;1:e337–47.
Mazzucchelli R, Crespi Villarias N, Perez Fernandez E, Durban Reguera ML, Garcia-Vadillo A, Quiros FJ, Guzon O, Rodriguez Caravaca G, Gil de Miguel A. Short-term association between outdoor air pollution and osteoporotic hip fracture. Osteoporos Int. 2018;29: 2231–2241.
Kheirouri S, Alizadeh M, Abad RMS, Barkabi-Zanjani S, Mesgari-Abbasi M. Effects of sulfur dioxide, ozone, and ambient air pollution on bone metabolism related biochemical parameters in a rat model. Environ Anal Heal Toxicol. 2020;35(4):e2020023.
Ranzani OT, Milà C, Kulkarni B, Kinra S, Tonne C. Association of ambient and household air pollution with bone mineral content among adults in peri-urban South India. JAMA Netw Open. 2020;3:e1918504–e1918504.
Qiao D, Pan J, Chen G, Xiang H, Tu R, Zhang X, Dong X, Wang Y, Luo Z, Tian H. Long-term exposure to air pollution might increase prevalence of osteoporosis in Chinese rural population. Environ Res. 2020;183:109264.
Adami G, Cattani G, Rossini M, Viapiana O, Olivi P, Orsolini G, Bertoldo E, Fracassi E, Gatti D, Fassio A. Association between exposure to fine particulate matter and osteoporosis: a population-based cohort study. Osteoporos Int. 2022;8(1):e002055.
Prada D, Crandall CJ, Kupsco A, Kioumourtzoglou M-A, Stewart JD, Liao D, Yanosky JD, Ramirez A, Wactawski-Wende J, Shen Y. Air pollution and decreased bone mineral density among Women’s Health Initiative participants. EClinicalMedicine. 2023;57:101864.
Qi W, Mei Z, Sun Z, Lin C, Lin J, Li J, Ji JS, Zheng Y. Exposure to Multiple Air Pollutants and the Risk of Fractures: A Large Prospective Population-Based Study. J Bone Miner Res. 2023;38:1549–59.
Yu X-H, Cao H-W, Bo L, Lei S-F, Deng F-Y. Air pollution, genetic factors and the risk of osteoporosis: A prospective study in the UK biobank. Front Public Heal. 2023;11:1119774.
Yang Y, Li R, Cai M, Wang X, Li H, Wu Y, Chen L, Zou H, Zhang Z, Li H. Ambient air pollution, bone mineral density and osteoporosis: results from a national population-based cohort study. Chemosphere. 2023;310:136871.
Qinwen G, Sijia Y, Yu Q, Jiali C, Wenhua Y, Sanduo L, Pinger W, Ran L, Lu Z, Guobo C, et al. Ambient PM2.5 Exposure and Bone Homeostasis: Analysis of UK Biobank Data and Experimental Studies in Mice and in Vitro. Environ Health Perspect. 2024;131:107002. https://doi.org/10.1289/EHP11646.
Zhang X, Yu S, Zhang F, Zhu S, Zhao G, Zhang X, Li T, Yu B, Zhu W, Li D. Association between traffic-related air pollution and osteoporotic fracture hospitalizations in inland and coastal areas: evidences from the central areas of two cities in Shandong Province. China Arch Osteoporos. 2023;18:96.
Jiang R, Qu Q, Wang Z, Luo F, Mou S. Association between air pollution and bone mineral density: a Mendelian randomization study. Arch Med Sci. 2024;20(4). https://doi.org/10.5114/aoms/192628.
Sung JH, Kim K, Cho Y, Choi S, Chang J, Kim SM, Kim SR, Lee G, Son JS, Park SM. Association of air pollution with osteoporotic fracture risk among women over 50 years of age. J Bone Miner Metab. 2020;38:839–47.
Oh TK, Song I-A. Exposure to air pollution and risk of hip fracture: a population-based cohort study with a 6-year follow-up in South Korea. J Occup Environ Med. 2020;62:1034–9.
Lin Y-H, Wang C-F, Chiu H, Lai B-C, Tu H-P, Wu P-Y, Huang J-C, Chen S-C. Air pollutants interaction and gender difference on bone mineral density T-score in Taiwanese adults. Int J Environ Res Public Health. 2020;17:9165.
Mousavibaygei SR, Bisadi A, ZareSakhvidi F. Outdoor air pollution exposure, bone mineral density, osteoporosis, and osteoporotic fractures: a systematic review and meta-analysis. Sci Total Environ. 2023;865:161117.
Prada D, López G, Solleiro-Villavicencio H, Garcia-Cuellar C, Baccarelli AA. Molecular and cellular mechanisms linking air pollution and bone damage. Environ Res. 2020;185:109465. https://doi.org/10.1016/j.envres.2020.109465.
Snega Priya P, Pratiksha Nandhini P, Arockiaraj J. A comprehensive review on environmental pollutants and osteoporosis: Insights into molecular pathways. Environ Res. 2023;237:117103. https://doi.org/10.1016/j.envres.2023.117103.
Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801.
Bodine PVN, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:33–9. https://doi.org/10.1007/s11154-006-9002-4.
Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. https://doi.org/10.1007/s11154-006-9001-5.
Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL–RANK interaction. J Exp Med. 2000;191:275–86.
Fu S, Thompson CL, Ali A, Wang W, Chapple JP, Mitchison HM, Beales PL, Wann AKT, Knight MM. Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthr Cartil. 2019;27:1064–74.
Verbruggen SW, Sittichokechaiwut A, Reilly GC. Osteocytes and Primary Cilia. Curr Osteoporos Rep. 2023;21(6):719–30.
Wann AKT, Knight MM. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell Mol Life Sci. 2012;69:2967–77.
Verbruggen SW, Nolan J, Duffy MP, Pearce OMT, Jacobs CR, Knight MM. A novel primary cilium-mediated mechanism through which osteocytes regulate metastatic behavior of both breast and prostate cancer cells. Adv Sci. 2024;11:2305842.
World Urbanization Prospects: The 2018 Revision. Kenya: UN; 2019. https://books.google.co.uk/books?id=Kp9AygEACAAJ.
Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol 2014;34(5).
Thompson CL, Fu S, Heywood HK, Knight MM, Thorpe SD. Mechanical stimulation: a crucial element of organ-on-chip models. Front Bioeng Biotechnol. 2020;8:602646.
Nolan J, Pearce OMT, Screen HRC, Knight MM, Verbruggen SW. Organ-on-a-Chip and Microfluidic Platforms for Oncology in the UK. Cancers (Basel). 2023;15:635.
Jain S. Down to the bone: there’s growing evidence that breathing polluted air increases the risk of osteoporosis. Science. 2024;385(6707):359–61.
Acknowledgements
This publication was supported by the U.K. Engineering and Physical Sciences Research Council (S.W.V.) [grant number EP/Y001842/1]. This work forms part of the research portfolio of the National Institute for Health Research Barts Biomedical Research Centre (NIHR203330). The authors declare that no competing financial interests exist.
Author information
Authors and Affiliations
Contributions
SV and MK developed the idea for the article. OA and SV performed the literature search, and OA performed the data analysis. OA, SV and MK drafted and critically revised the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allen, O., Knight, M.M. & Verbruggen, S.W. Air Pollution and Osteoporosis. Curr Osteoporos Rep (2024). https://doi.org/10.1007/s11914-024-00889-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11914-024-00889-9