Abstract
Purpose of Review
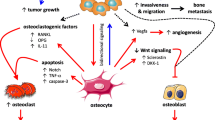
While the function of osteocytes under physiologic conditions is well defined, their role and involvement in cancer disease remains relatively unexplored, especially in a context of non-bone metastatic cancer. This review will focus on describing the more advanced knowledge regarding the interactions between osteocytes and cancer.
Recent Findings
We will discuss the involvement of osteocytes in the onset and progression of osteosarcoma, with the common bone cancers, as well as the interaction that is established between osteocytes and multiple myeloma. Mechanisms responsible for cancer dissemination to bone, as frequently occur with advanced breast and prostate cancers, will be reviewed. While a role for osteocytes in the stimulation and proliferation of cancer cells has been reported, protective effects of osteocytes against bone colonization have been described as well, thus increasing ambiguity regarding the role of osteocytes in cancer progression and dissemination. Lastly, supporting the idea that skeletal defects can occur also in the absence of direct cancer dissemination or osteolytic lesions directly adjacent to the bone, our recent findings will be presented showing that in the absence of bone metastases, the bone microenvironment and, particularly, osteocytes, can manifest a clear and dramatic response to the distant, non-metastatic tumor.
Summary
Our observations support new studies to clarify whether treatments designed to preserve the osteocytes can be combined with traditional anticancer therapies, even when bone is not directly affected by tumor growth.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone. 2015;75:144–50.
Cui YX, Evans BA, Jiang WG. New roles of osteocytes in proliferation, migration and invasion of breast and prostate cancer cells. Anticancer Res. 2016;36:1193–201.
Liu S, Fan Y, Chen A, Jalali A, Minami K, Ogawa K, Nakshatri H, Li BY, Yokota H. Osteocyte-driven downregulation of snail restrains effects of Drd2 inhibitors on mammary tumor cells. Cancer Res. 2018;78:3865–76.
Wang W, Yang X, Dai J, Lu Y, Zhang J, Keller ET. Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene. 2019;38:4540–59.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more. Endocr Rev. 2013;34:658–90.
Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16:2017–26.
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5.
Knothe Tate ML, Steck R, Forwood MR, Niederer P. In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Biol. 2000;203:2737–45.
Yuan H, Jiang W, Chen Y, Kim BYS. Study of osteocyte behavior by high-resolution intravital imaging following photo-induced ischemia. Molecules. 2018;23:2874.
Wein MN. Parathyroid hormone signaling in osteocytes. JBMR Plus. 2018;2:22–30.
Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37.
Pike JW, Lee SM, Meyer MB. Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. Bonekey Rep. 2014;3:482.
McDonald MM, Delgado-Calle J. Sclerostin: an emerging target for the treatment of cancer-induced bone disease. Curr Osteoporos Rep. 2017;15:532–41.
Ming J, Cronin SJF, Penninger JM. Targeting the RANKL/RANK/OPG axis for cancer therapy. Front Oncol. 2020;10:1283.
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59:99–107.
Blair JM, Zhou H, Seibel MJ, Dunstan CR. Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat Clin Pract Oncol. 2006;3:41–9.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–4.
Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, Bonewald L, Manolagas SC, O’Brien CA. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One. 2015;10:e0138189.
Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020;82:485–506.
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–7.
Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol. 2005;25:4946–55.
Chen J. Long F: beta-catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. J Bone Miner Res. 2013;28:1160–9.
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–9.
Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15:928–35.
Ray S, Khassawna TE, Sommer U, Thormann U, Wijekoon ND, Lips K, Heiss C, Alt V. Differences in expression of Wnt antagonist Dkk1 in healthy versus pathological bone samples. J Microsc. 2017;265:111–20.
Colditz J, Thiele S, Baschant U, Garbe AI, Niehrs C, Hofbauer LC, Rauner M. Osteogenic Dkk1 mediates glucocorticoid-induced but not arthritis-induced bone loss. J Bone Miner Res. 2019;34:1314–23.
Witcher PC, Miner SE, Horan DJ, Bullock WA, Lim KE, Kang KS, Adaniya AL, Ross RD, Loots GG, Robling AG. Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight. 2018;3:e98673.
Li X, Kordsmeier J, Xiong J. New advances in osteocyte mechanotransduction. Curr Osteoporos Rep. 2021;19:101–6.
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–17.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75.
Storlino G, Colaianni G, Sanesi L, Lippo L, Brunetti G, Errede M, Colucci S, Passeri G, Grano M. Irisin prevents disuse-induced osteocyte apoptosis. J Bone Miner Res. 2020;35:766–75.
Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman VA, Novick SJ, et al. Irisin mediates effects on bone and Fat via alphaV integrin receptors. Cell. 2019;178:507–8.
Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jahn K, Yi J, Zhou J, Brotto M, Bonewald LF. Beta-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018;22:1531–44.
Pagnotti GM, Adler BJ, Green DE, Chan ME, Frechette DM, Shroyer KR, Beamer WG, Rubin J, Rubin CT. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone. 2012;51:570–7.
Lynch ME, Brooks D, Mohanan S, Lee MJ, Polamraju P, Dent K, Bonassar LJ, van der Meulen MC, Fischbach C. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J Bone Miner Res. 2013;28:2357–67.
Ma YV, Xu L, Mei X, Middleton K, You L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J Cell Biochem. 2018;120:7590–601.
Sheill G, Guinan EM, Peat N, Hussey J. Considerations for exercise prescription in patients with bone metastases: a comprehensive narrative review. PM R. 2018;10:843–64.
Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27:1018–29.
Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27:1936–50.
Kogawa M, Wijenayaka AR, Ormsby RT, Thomas GP, Anderson PH, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J Bone Miner Res. 2013;28:2436–48.
Belanger LF, Belanger C, Semba T. Technical approaches leading to the concept of osteocytic osteolysis. Clin Orthop Relat Res. 1967;54:187–96.
Tsourdi E, Jahn K, Rauner M, Busse B, Bonewald LF. Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact. 2018;18:292–303.
Hongo H, Hasegawa T, Saito M, Tsuboi K, Yamamoto T, Sasaki M, Abe M, Henrique Luiz de Freitas P, Yurimoto H, Udagawa N, et al. Osteocytic osteolysis in PTH-treated wild-type and Rankl(-/-) mice examined by transmission electron microscopy, atomic force microscopy, and isotope microscopy. J Histochem Cytochem. 2020;68:651–68.
Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, Gludovatz B, Walsh F, Regan JN, Messina S, Evans DS, Lang TF, Zhang B, Ritchie RO, Mohammad KS, Alliston T. Osteocyte-intrinsic TGF-beta signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;21:2585–96.
Rolvien T, Krause M, Jeschke A, Yorgan T, Puschel K, Schinke T, Busse B, Demay MB, Amling M. Vitamin D regulates osteocyte survival and perilacunar remodeling in human and murine bone. Bone. 2017;103:78–87.
Davey RA, Clarke MV, Golub SB, Russell PK, Zajac JD. The calcitonin receptor regulates osteocyte lacunae acidity during lactation in mice. J Endocrinol. 2021;249:31–41.
Zambonin Zallone A, Teti A, Primavera MV, Pace G. Mature osteocytes behaviour in a repletion period: the occurrence of osteoplastic activity. Basic Appl Histochem. 1983;27:191–204.
•• Pin F, Prideaux M, Huot JR, Essex AL, Plotkin LI, Bonetto A, Bonewald LF. Non-bone metastatic cancers promote osteocyte-induced bone destruction. Cancer Lett. 2021;520:80–90. This study reports for the first time osteocytic osteolysis and extensive osteocyte cell death in three different preclinical models of non-bone metastatic tumors
Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT J. 2018;4:12.
Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–35.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012:704872–10.
Lin YH, Jewell BE, Gingold J, Lu L, Zhao R, Wang LL, Lee DF. Osteosarcoma: molecular pathogenesis and iPSC modeling. Trends Mol Med. 2017;23:737–55.
Mutsaers AJ, Walkley CR. Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone. 2014;62:56–63.
Sottnik JL, Campbell B, Mehra R, Behbahani-Nejad O, Hall CL, Keller ET. Osteocytes serve as a progenitor cell of osteosarcoma. J Cell Biochem. 2014;115:1420–9.
Kashima TG, Dongre A, Oppermann U, Athanasou NA. Dentine matrix protein 1 (DMP-1) is a marker of bone-forming tumours. Virchows Arch. 2013;462:583–91.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, Dimopoulos MA. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8:7.
Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B, Bonomini S, Martella E, Agnelli L, Neri A, Ceccarelli F, Palumbo C. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 2012;26:1391–401.
Trotter TMFM, Gibson JT, Peker D, Javed A, Yang Y. Osteocyte apoptosis attracts myeloma cells to bone and supports progression through regulation of the bone marrow microenvironment. Blood. 2016;128:484.
Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, Delimpasi S, Pouli A, Meletis J, Kastritis E, Zervas K, Dimopoulos MA. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012;131:1466–71.
McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL, Pettitt JA, Simic MK, Cheng TL, Morse A, et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017;129:3452–64.
Delgado-Calle J, Anderson J, Cregor MD, Condon KW, Kuhstoss SA, Plotkin LI, Bellido T, Roodman GD. Genetic deletion of Sost or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia. 2017;31:2686–94.
• Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, Yoneda T, Mohammad KS, Plotkin LI, Roodman GD, Bellido T. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76:1089–100. This is an important study showing that osteocytes and MM cells present a physically bidirectional interaction that regulates the Notch pathway, ultimately promoting MM cell growth
• Mulcrone PL, Edwards SKE, Petrusca DN, Haneline LS, Delgado-Calle J, Roodman GD. Osteocyte Vegf-a contributes to myeloma-associated angiogenesis and is regulated by Fgf23. Sci Rep. 2020;10:17319. This study provides evidence that osteocytes increase bone marrow angiogenesis in MM by producing Vegf-a and Fgf23 in response to hypoxia
Suvannasankha A, Tompkins DR, Edwards DF, Petyaykina KV, Crean CD, Fournier PG, Parker JM, Sandusky GE, Ichikawa S, Imel EA, Chirgwin JM. FGF23 is elevated in multiple myeloma and increases heparanase expression by tumor cells. Oncotarget. 2015;6:19647–60.
Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, Brufsky AM, Clezardin P, Croucher PI, Gralow JR, Hadji P, et al. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat Rev. 2010;36:615–20.
Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–25.
Huang JF, Shen J, Li X, Rengan R, Silvestris N, Wang M, Derosa L, Zheng X, Belli A, Zhang XL, Li YM, Wu A. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann Transl Med. 2020;8:482.
Maroni P, Bendinelli P. Bone, a secondary growth site of breast and prostate carcinomas: role of osteocytes. Cancers (Basel). 2020;12:1812.
Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med. 2008;10:e7.
Stinson JC. The ailing mythical osteocyte. Med Hypotheses. 1975;1:186–90.
Kerschnitzki M, Kollmannsberger P, Burghammer M, Duda GN, Weinkamer R, Wagermaier W, Fratzl P. Architecture of the osteocyte network correlates with bone material quality. J Bone Miner Res. 2013;28:1837–45.
Hemmatian H, Conrad S, Furesi G, Mletzko K, Krug J, Faila AV, Kuhlmann JD, Rauner M, Busse B, Jahn-Rickert K. Reorganization of the osteocyte lacuno-canalicular network characteristics in tumor sites of an immunocompetent murine model of osteotropic cancers. Bone. 2021;152:116074.
Sano T, Sun X, Feng Y, Liu S, Hase M, Fan Y, Zha R, Wu D, Aryal UK, Li BY, Sudo A, Yokota H. Inhibition of the growth of breast cancer-associated brain tumors by the osteocyte-derived conditioned medium. Cancers (Basel). 2021;13:1061.
Custodio-Santos T, Videira M, Brito MA. Brain metastasization of breast cancer. Biochim Biophys Acta Rev Cancer. 2017;1868:132–47.
Zhou JZ, Riquelme MA, Gu S, Kar R, Gao X, Sun L, Jiang JX. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene. 2016;35:5597–607.
Fan Y, Jalali A, Chen A, Zhao X, Liu S, Teli M, Guo Y, Li F, Li J, Siegel A, Yang L, Liu J, Na S, Agarwal M, Robling AG, Nakshatri H, Li BY, Yokota H. Skeletal loading regulates breast cancer-associated osteolysis in a loading intensity-dependent fashion. Bone Res. 2020;8:9.
Wang W, Sarazin BA, Kornilowicz G, Lynch ME. Mechanically-loaded breast cancer cells modify osteocyte mechanosensitivity by secreting factors that increase osteocyte dendrite formation and downstream resorption. Front Endocrinol (Lausanne). 2018;9:352.
Dwivedi A, Kiely PA, Hoey DA. Mechanically stimulated osteocytes promote the proliferation and migration of breast cancer cells via a potential CXCL1/2 mechanism. Biochem Biophys Res Commun. 2021;534:14–20.
Choudhary S, Ramasundaram P, Dziopa E, Mannion C, Kissin Y, Tricoli L, Albanese C, Lee W, Zilberberg J. Human ex vivo 3D bone model recapitulates osteocyte response to metastatic prostate cancer. Sci Rep. 2018;8:17975.
Pagnotti GM, Chan ME, Adler BJ, Shroyer KR, Rubin J, Bain SD, Rubin CT. Low intensity vibration mitigates tumor progression and protects bone quantity and quality in a murine model of myeloma. Bone. 2016;90:69–79.
Zhou JZ, Riquelme MA, Gao X, Ellies LG, Sun LZ, Jiang JX. Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncogene. 2015;34:1831–42.
Sottnik JL, Dai J, Zhang H, Campbell B, Keller ET. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 2015;75:2151–8.
Wang S, Pei S, Wasi M, Parajuli A, Yee A, You L, Wang L. Moderate tibial loading and treadmill running, but not overloading, protect adult murine bone from destruction by metastasized breast cancer. Bone. 2021;153:116100.
Huang M, Liu H, Zhu L, Li X, Li J, Yang S, Liu D, Song X, Yokota H, Zhang P. Mechanical loading attenuates breast cancer-associated bone metastasis in obese mice by regulating the bone marrow microenvironment. J Cell Physiol. 2021;236:6391–406.
Liu S, Wu D, Sun X, Fan Y, Zha R, Jalali A, Teli M, Sano T, Siegel A, Sudo A, Agarwal M, Robling A, Li BY, Yokota H. Mechanical stimulations can inhibit local and remote tumor progression by downregulating WISP1. FASEB J. 2020;34:12847–59.
Wu D, Fan Y, Liu S, Woollam MD, Sun X, Murao E, Zha R, Prakash R, Park C, Siegel AP, Liu J, Agarwal M, Li BY, Yokota H. Loading-induced antitumor capability of murine and human urine. FASEB J. 2020;34:7578–92.
Santos L, Ugun-Klusek A, Coveney C, Boocock DJ. Multiomic analysis of stretched osteocytes reveals processes and signalling linked to bone regeneration and cancer. NPJ Regen Med. 2021;6:32.
Bonetto A, Kays JK, Parker VA, Matthews RR, Barreto R, Puppa MJ, Kang KS, Carson JA, Guise TA, Mohammad KS, Robling AG, Couch ME, Koniaris LG, Zimmers TA. Differential bone loss in mouse models of colon cancer cachexia. Front Physiol. 2016;7:679.
Pin F, Barreto R, Kitase Y, Mitra S, Erne CE, Novinger LJ, Zimmers TA, Couch ME, Bonewald LF, Bonetto A. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: a new model for the study of cancer cachexia. J Cachexia Sarcopenia Muscle. 2018;9:685–700.
Berent TE, Dorschner JM, Craig TA, Drake MT, Westendorf JJ, Kumar R. Lung tumor cells inhibit bone mineralization and osteoblast activity. Biochem Biophys Res Commun. 2019;519:566–71.
Dumanskiy YV, Syniachenko OV, Stepko PA, Taktashov GS, Chernyshova OY, Stoliarova OY. The state of bone metabolism in lung cancer patients. Exp Oncol. 2018;40:136–9.
Hung YC, Yeh LS, Chang WC, Lin CC, Kao CH. Prospective study of decreased bone mineral density in patients with cervical cancer without bone metastases: a preliminary report. Jpn J Clin Oncol. 2002;32:422–4.
Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector T. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. 1999;79:1179–81.
Funding
This study was supported by the Department of Surgery and the Department of Otolaryngology — Head & Neck Surgery at Indiana University, by a grant from the NIH/NIA (PO1AG039355) to LFB, and by grants from the NIH/NIAMS (R01AR079379), Showalter Research Trust, V Foundation for Cancer Research (V2017-021), and American Cancer Society (Research Scholar Grant 132013-RSG-18-010-01-CCG) to AB
Author information
Authors and Affiliations
Contributions
FP, MP, LFB, and AB conceived the content of the manuscript; FP and MP wrote the manuscript; LFB and AB edited and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteocytes
Rights and permissions
About this article
Cite this article
Pin, F., Prideaux, M., Bonewald, L.F. et al. Osteocytes and Cancer. Curr Osteoporos Rep 19, 616–625 (2021). https://doi.org/10.1007/s11914-021-00712-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-021-00712-9