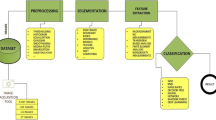
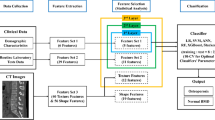
Abstract
Purpose of Review
In this paper, we discuss how recent advancements in image processing and machine learning (ML) are shaping a new and exciting era for the osteoporosis imaging field. With this paper, we want to give the reader a basic exposure to the ML concepts that are necessary to build effective solutions for image processing and interpretation, while presenting an overview of the state of the art in the application of machine learning techniques for the assessment of bone structure, osteoporosis diagnosis, fracture detection, and risk prediction.
Recent Findings
ML effort in the osteoporosis imaging field is largely characterized by “low-cost” bone quality estimation and osteoporosis diagnosis, fracture detection, and risk prediction, but also automatized and standardized large-scale data analysis and data-driven imaging biomarker discovery.
Summary
Our effort is not intended to be a systematic review, but an opportunity to review key studies in the recent osteoporosis imaging research landscape with the ultimate goal of discussing specific design choices, giving the reader pointers to possible solutions of regression, segmentation, and classification tasks as well as discussing common mistakes.






Similar content being viewed by others
Data availability
Not applicable.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
van Oostwaard M Osteoporosis and the nature of fragility fracture: an overview. In: Hertz K, Santy-Tomlinson J, editors. Fragility fracture nursing: holistic care and management of the orthogeriatric patient. Cham (CH)2018. p. 1-13.
Lewiecki EM, Lane NE. Common mistakes in the clinical use of bone mineral density testing. Nat Clin Pract Rheumatol. 2008;4(12):667–74. https://doi.org/10.1038/ncprheum0928.
Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, Reid DM, Selby P, Thompson F, Thurston A, Vine N, National Osteoporosis Guideline G. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. https://doi.org/10.1007/s11657-017-0324-5.
Han W, Qin L, Bay C, Chen X, Yu KH, Miskin N, Li A, Xu X, Young G. Deep transfer learning and radiomics feature prediction of survival of patients with high-grade gliomas. AJNR Am J Neuroradiol. 2020;41(1):40–8. https://doi.org/10.3174/ajnr.A6365.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44. https://doi.org/10.1038/nature14539.
Bengio Y, Courville A, Vincent P. Representation learning: a review and new perspectives. IEEE Trans Pattern Anal Mach Intell. 2013;35(8):1798–828. https://doi.org/10.1109/TPAMI.2013.50.
Bengio Y, Lee H. Editorial introduction to the neural networks special issue on deep learning of representations. Neural Netw. 2015;64:1–3. https://doi.org/10.1016/j.neunet.2014.12.006.
Smets J, Shevroja E, Hugle T, Leslie WD, Hans D. Machine learning solutions for osteoporosis-a review. J Bone Miner Res. 2021. https://doi.org/10.1002/jbmr.4292.
Fang Y, Li W, Chen X, Chen K, Kang H, Yu P, Zhang R, Liao J, Hong G, Li S. Opportunistic osteoporosis screening in multi-detector CT images using deep convolutional neural networks. Eur Radiol. 2021;31(4):1831–42. https://doi.org/10.1007/s00330-020-07312-8This is a fully automatic pipeline for vertebral segmentation and BMD assessment for opportunistic osteoporosis screening. Well-designed and relevant study.
Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. Medical Image Computing and Computer-Assisted Intervention, Pt Iii. 2015;9351:234-41. doi: https://doi.org/10.1007/978-3-319-24574-4_28.
Byra M, Wu M, Zhang X, Jang H, Ma YJ, Chang EY, Shah S, Du J. Knee menisci segmentation and relaxometry of 3D ultrashort echo time cones MR imaging using attention U-Net with transfer learning. Magn Reson Med. 2020;83(3):1109–22. https://doi.org/10.1002/mrm.27969.
Bagheri MH, Roth H, Kovacs W, Yao J, Farhadi F, Li X, Summers RM. Technical and clinical factors affecting success rate of a deep learning method for pancreas segmentation on CT. Acad Radiol. 2019. https://doi.org/10.1016/j.acra.2019.08.014
Curtis SL, Norman BP, Milan AM, Gallagher JA, Olsson B, Ranganath LR, Roberts NB. Interference of hydroxyphenylpyruvic acid, hydroxyphenyllactic acid and tyrosine on routine serum and urine clinical chemistry assays; implications for biochemical monitoring of patients with alkaptonuria treated with nitisinone. Clin Biochem. 2019;71:24–30. https://doi.org/10.1016/j.clinbiochem.2019.06.010.
Pang S, Su Z, Leung S, Nachum IB, Chen B, Feng Q, Li S. Direct automated quantitative measurement of spine by cascade amplifier regression network with manifold regularization. Med Image Anal. 2019;55:103–15. https://doi.org/10.1016/j.media.2019.04.012.
Xiao P, Zhang T, Dong XN, Han Y, Huang Y, Wang X. Prediction of trabecular bone architectural features by deep learning models using simulated DXA images. Bone Rep. 2020;13:100295. https://doi.org/10.1016/j.bonr.2020.100295.
Ruder S. An overview of multi-task learning in deep neural networks. arXiv preprint arXiv:170605098, 2017. 2017.
Lee KS, Jung SK, Ryu JJ, Shin SW, Choi J. Evaluation of transfer learning with deep convolutional neural networks for screening osteoporosis in dental panoramic radiographs. J Clin Med. 2020;9(2). https://doi.org/10.3390/jcm9020392.
Miotto R, Wang F, Wang S, Jiang X, Dudley JT. Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform. 2018;19(6):1236–46. https://doi.org/10.1093/bib/bbx044.
Warman A, Warman P, Sharma A, Parikh P, Warman R, Viswanadhan N, Chen L, Mohapatra S, Mohapatra S, Sapiro G. Interpretable artificial intelligence for COVID-19 diagnosis from chest CT reveals specificity of ground-glass opacities. medRxiv. 2020. https://doi.org/10.1101/2020.05.16.20103408.
Wang F, Kaushal R, Khullar D. Should health care demand interpretable artificial intelligence or accept "black box" medicine? Ann Intern Med. 2020;172(1):59–60. https://doi.org/10.7326/M19-2548.
Rotemberg V, Halpern A. Towards 'interpretable' artificial intelligence for dermatology. Br J Dermatol. 2019;181(1):5–6. https://doi.org/10.1111/bjd.18038.
Yamamoto N, Sukegawa S, Kitamura A, Goto R, Noda T, Nakano K, Takabatake K, Kawai H, Nagatsuka H, Kawasaki K, Furuki Y, Ozaki T. Deep learning for osteoporosis classification using hip radiographs and patient clinical covariates. Biomolecules. 2020;10(11). https://doi.org/10.3390/biom10111534.
Zhang B, Yu K, Ning Z, Wang K, Dong Y, Liu X, Liu S, Wang J, Zhu C, Yu Q, Duan Y, Lv S, Zhang X, Chen Y, Wang X, Shen J, Peng J, Chen Q, Zhang Y, et al. Deep learning of lumbar spine X-ray for osteopenia and osteoporosis screening: a multicenter retrospective cohort study. Bone. 2020;140:115561. https://doi.org/10.1016/j.bone.2020.115561.
Olczak J, Fahlberg N, Maki A, Razavian AS, Jilert A, Stark A, Skoldenberg O, Gordon M. Artificial intelligence for analyzing orthopedic trauma radiographs. Acta Orthop. 2017;88(6):581–6. https://doi.org/10.1080/17453674.2017.1344459.
Lindsey R, Daluiski A, Chopra S, Lachapelle A, Mozer M, Sicular S, Hanel D, Gardner M, Gupta A, Hotchkiss R, Potter H. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci U S A. 2018;115(45):11591–6. https://doi.org/10.1073/pnas.1806905115.
Badgeley MA, Zech JR, Oakden-Rayner L, Glicksberg BS, Liu M, Gale W, MV MC, Percha B, Snyder TM, Dudley JT. Deep learning predicts hip fracture using confounding patient and healthcare variables. NPJ Digit Med. 2019;2:31. https://doi.org/10.1038/s41746-019-0105-1Largest study on fracture detection. The in-depth analysis of the feature is interesting and well executed. It proposed solution for multimodal data integration: imaging and clinical.
Krogue JD, Cheng KV, Hwang K, Toogood P, Meinberg E, Geiger E, Zaid M, Ozhinsky E, Majumdar S, Pedoia V. Automatic Hip fracture identification and functional subclassification with deep learning. Radiol: Artif Intell. 2020;2(2).
Murata K, Endo K, Aihara T, Suzuki H, Sawaji Y, Matsuoka Y, Nishimura H, Takamatsu T, Konishi T, Maekawa A, Yamauchi H, Kanazawa K, Endo H, Tsuji H, Inoue S, Fukushima N, Kikuchi H, Sato H, Yamamoto K. Artificial intelligence for the detection of vertebral fractures on plain spinal radiography. Sci Rep. 2020;10(1):20031. https://doi.org/10.1038/s41598-020-76866-w.
Almog YA, Rai A, Zhang P, Moulaison A, Powell R, Mishra A, Weinberg K, Hamilton C, Oates M, McCloskey E, Cummings SR. Deep learning with electronic health records for short-term fracture risk identification: crystal bone algorithm development and validation. J Med Internet Res. 2020;22(10):e22550. https://doi.org/10.2196/22550.
Muller R. Hierarchical microimaging of bone structure and function. Nat Rev Rheumatol. 2009;5(7):373–81. https://doi.org/10.1038/nrrheum.2009.107.
Cheung AM, Adachi JD, Hanley DA, Kendler DL, Davison KS, Josse R, Brown JP, Ste-Marie LG, Kremer R, Erlandson MC, Dian L, Burghardt AJ, Boyd SK. High-resolution peripheral quantitative computed tomography for the assessment of bone strength and structure: a review by the Canadian Bone Strength Working Group. Curr Osteoporos Rep. 2013;11(2):136–46. https://doi.org/10.1007/s11914-013-0140-9.
Langsetmo L, Peters KW, Burghardt AJ, Ensrud KE, Fink HA, Cawthon PM, Cauley JA, Schousboe JT, Barrett-Connor E, Orwoll ES. Osteoporotic Fractures in Men Study Research G. Volumetric bone mineral density and failure load of distal limbs predict incident clinical fracture independent HR-pQCT BMD and failure load predicts incident clinical fracture of FRAX and clinical risk factors among older men. J Bone Miner Res. 2018;33(7):1302–11. https://doi.org/10.1002/jbmr.3433.
Mikolajewicz N, Bishop N, Burghardt AJ, Folkestad L, Hall A, Kozloff KM, Lukey PT, Molloy-Bland M, Morin SN, Offiah AC, Shapiro J, van Rietbergen B, Wager K, Willie BM, Komarova SV, Glorieux FH. HR-pQCT measures of bone microarchitecture predict fracture: systematic review and meta-analysis. J Bone Miner Res. 2020;35(3):446–59. https://doi.org/10.1002/jbmr.3901.
Li Y, Sixou B, Burghard B, Peyrin F. Investigation of semi-coupled dictionary learning in 3-D super resolution HR-PQCT imaging. IEEE Transactions on Radiation and Plasma Medical Sciences. 2019;3(2).
Guha I, Nadeem SA, You C, Zhang X, Levy SM, Wang G, Torner JC, Saha PK. Deep learning based high-resolution reconstruction of trabecular bone microstructures from low-resolution CT scans using GAN-CIRCLE. Proc SPIE Int Soc Opt Eng. 2020:11317. https://doi.org/10.1117/12.2549318.
Karasik D, Demissie S, Zhou Y, Lu D, Broe KE, Bouxsein ML, Cupples LA, Kiel DP. Heritability and genetic correlations for bone microarchitecture: the Framingham study families. J Bone Miner Res. 2017;32(1):106–14. https://doi.org/10.1002/jbmr.2915.
Biver E, Durosier-Izart C, Chevalley T, van Rietbergen B, Rizzoli R, Ferrari S. Evaluation of radius microstructure and areal bone mineral density improves fracture prediction in postmenopausal women. J Bone Miner Res. 2018;33(2):328–37. https://doi.org/10.1002/jbmr.3299.
Atkinson EJ, Therneau TM, Melton LJ 3rd, Camp JJ, Achenbach SJ, Amin S, Khosta S. Assessing fracture risk using gradient boosting machine (GBM) models. J Bone Miner Res. 2012;27(6):1397–404. https://doi.org/10.1002/jbmr.1577This study shows the usage of statistical multi-parametric modeling for fracture discrimination interesting applications and extremely relevant for model interpretability.
Treece G, Gee A. Cortical bone mapping: measurement and statistical analysis of localised skeletal changes. Curr Osteoporos Rep. 2018;16(5):617–25. https://doi.org/10.1007/s11914-018-0475-3.
Carballido-Gamio J, Yu A, Wang L, Su Y, Burghardt AJ, Lang TF, Cheng X. Hip fracture discrimination based on statistical multi-parametric modeling (SMPM). Ann Biomed Eng. 2019;47(11):2199–212. https://doi.org/10.1007/s10439-019-02298-x.
Treece GM, Gee AH, Tonkin C, Ewing SK, Cawthon PM, Black DM, Poole KE. Osteoporotic Fractures in Men S. Predicting hip fracture type with cortical bone mapping (CBM) in the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res. 2015;30(11):2067–77. https://doi.org/10.1002/jbmr.2552.
Carballido-Gamio J, Bonaretti S, Kazakia GJ, Khosla S, Majumdar S, Lang TF, Burghardt AJ. Statistical parametric mapping of HR-pQCT images: a tool for population-based local comparisons of micro-scale bone features. Ann Biomed Eng. 2017;45(4):949–62. https://doi.org/10.1007/s10439-016-1754-8.
Kogan F, Broski SM, Yoon D, Gold GE. Applications of PET-MRI in musculoskeletal disease. J Magn Reson Imaging. 2018;48(1):27–47. https://doi.org/10.1002/jmri.26183.
Kogan F, Fan AP, Monu U, Iagaru A, Hargreaves BA, Gold GE. Quantitative imaging of bone-cartilage interactions in ACL-injured patients with PET-MRI. Osteoarthr Cartil. 2018;26(6):790–6. https://doi.org/10.1016/j.joca.2018.04.001.
Tibrewala R, Pedoia V, Bucknor M, Majumdar S. Principal component analysis of simultaneous PET-MRI reveals patterns of bone-cartilage interactions in osteoarthritis. J Magn Reson Imaging. 2020;52:1462–74. https://doi.org/10.1002/jmri.27146.
Wu PH, Gibbons M, Foreman SC, Carballido-Gamio J, Han M, Krug R, Liu J, Link TM, Kazakia GJ. Cortical bone vessel identification and quantification on contrast-enhanced MR images. Quant Imaging Med Surg. 2019;9(6):928–41. https://doi.org/10.21037/qims.2019.05.23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors do not have conflicts of interests with the material presented in this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Pedoia, V., Caliva, F., Kazakia, G. et al. Augmenting Osteoporosis Imaging with Machine Learning. Curr Osteoporos Rep 19, 699–709 (2021). https://doi.org/10.1007/s11914-021-00701-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-021-00701-y