Abstract
Purpose of Review
In this review, we provide a recent update on bioenergetic pathways in osteocytes and identify potential future areas of research interest. Studies have identified a role for regulation of bone formation and bone resorption through osteocyte mechanosensing and osteocyte secreted factors. Nevertheless, there is a paucity of studies on the bioenergetics and energy metabolism of osteocytes, which are required for the regulation of bone remodeling.
Recent Findings
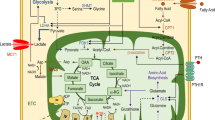
Osteocytes are cells of the osteoblast lineage embedded in bone. The osteocyte lacunocanalicular network within the skeletal matrix is exposed to a unique hypoxic environment. Therefore, the bioenergetic requirements of these cells could differ from other bone cells due to its location in the ossified matrix and its role in bone regulation transduced by mechanical signals. Recent findings highlighted in this review provide some evidence that metabolism of these cells is dependent on their location due to the substrates present in the microenvironment and metabolic cues from stress pathways. Both glycolysis (glucose metabolism) and oxidative phosphorylation (mitochondrial dynamics, ROS generation) affect osteocyte function and viability.
Summary
In this review, we provide evidence that is currently available about information regarding bioenergetics pathways in osteocytes. We discuss published studies showing a role for hypoxia-driven glucose metabolism in regulating osteocyte bioenergetics. We also provide information on various substrates that osteocytes could utilize to fuel energetic needs, namely pyruvate, amino acids, and fatty acids. This is based on some preliminary experimental evidence that is available in literature. The role of parathyroid hormone PTH and parathryoid hormone-related peptide PTHrP in bone anabolism and resorption, along with regulation of metabolic pathways in the cells of the skeletal niche, needs to be explored further. Mitochondrial metabolism has a role in osteocyte bioenergetics through substrate utilization, location of the osteocyte in the bone cortex, and mitochondrial biogenesis. While there are limitations in studying metabolic flux in traditional cell lines, there are now novel cell lines and sophisticated tools available to study osteocyte bioenergetics to help harness its potential in vivo in the future.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as:•• Of major importance
Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys. 2014;561:3–12.
Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone. 2015;75:144–50.
Bakker AD, Kulkarni RN, Klein-Nulend J, Lems WF. IL-6 alters osteocyte signaling toward osteoblasts but not osteoclasts. J Dent Res. 2014;93(4):394–9.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... And more. Endocr Rev. 2013;34(5):658–90.
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–76.
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24(10):1651–61.
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–7.
Balemans W, Ebeling M, Patel N, van Hul E, Olson P, Dioszegi M, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10(5):537–44.
Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017;114(52):E11238–47.
McClung MR, Brown JP, Diez-Perez A, Resch H, Caminis J, Meisner P, et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33(8):1397–406.
Geusens P, Oates M, Miyauchi A, Adachi JD, Lazaretti-Castro M, Ebeling PR, et al. The effect of 1 year of romosozumab on the incidence of clinical vertebral fractures in postmenopausal women with osteoporosis: results from the FRAME study. JBMR Plus. 2019;3(10):e10211.
Nampei A, Hashimoto J, Hayashida K, Tsuboi H, Shi K, Tsuji I, et al. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J Bone Miner Metab. 2004;22(3):176–84.
Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, et al. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16(11):2017–26.
Ubaidus S, Li M, Sultana S, de Freitas PHL, Oda K, Maeda T, et al. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc. 2009;58(6):381–92.
Miyagawa K, Yamazaki M, Kawai M, Nishino J, Koshimizu T, Ohata Y, et al. Dysregulated gene expression in the primary osteoblasts and osteocytes isolated from hypophosphatemic Hyp mice. PLoS One. 2014;9(4):e93840.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4.
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):e25900.
Jähn K, et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017;32(8):1761–72 This interesting study points to a potential role for glycolytic pathways in regulating osteolytic osteolysis.
Goldring SR. The osteocyte: key player in regulating bone turnover. RMD Open. 2015;1(Suppl 1):e000049.
Brun J, Berthou F, Trajkovski M, Maechler P, Foti M, Bonnet N. Bone regulates browning and energy metabolism through mature osteoblast/osteocyte PPARgamma expression. Diabetes. 2017;66(10):2541–54.
Sato M, Asada N, Kawano Y, Wakahashi K, Minagawa K, Kawano H, et al. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab. 2013;18(5):749–58.
Frikha-Benayed D, Basta-Pljakic J, Majeska RJ, Schaffler MB. Regional differences in oxidative metabolism and mitochondrial activity among cortical bone osteocytes. Bone. 2016;90:15–22.
Guo D, Keightley A, Guthrie J, Veno PA, Harris SE, Bonewald LF. Identification of osteocyte-selective proteins. Proteomics. 2010;10(20):3688–98.
Zahm AM, Bohensky J, Adams CS, Shapiro IM, Srinivas V. Bone cell autophagy is regulated by environmental factors. Cells Tissues Organs. 2011;194(2–4):274–8.
Loots GG, et al. Vhl deficiency in osteocytes produces high bone mass and hematopoietic defects. Bone. 2018;116:307–14 This is the first study to target the oxygen sensing system in osteocytes.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5.
Yellowley CE, Genetos DC. Hypoxia signaling in the skeleton: implications for bone health. Current osteoporosis reports. 2019;17(1):26–35.
Stegen S, Stockmans I, Moermans K, Thienpont B, Maxwell PH, Carmeliet P, et al. Osteocytic oxygen sensing controls bone mass through epigenetic regulation of sclerostin. Nat Commun. 2018;9(1):2557.
Yao Q, Khan MP, Merceron C, LaGory EL, Tata Z, Mangiavini L, et al. Suppressing mitochondrial respiration is critical for hypoxia tolerance in the fetal growth plate. Dev Cell. 2019;49(5):748–63 e7.
Madhu V, Boneski PK, Silagi E, Qiu Y, Kurland I, Guntur AR, et al. Hypoxic regulation of mitochondrial metabolism and mitophagy in nucleus pulposus cells is dependent on HIF-1alpha-BNIP3 Axis. J Bone Miner Res. 2020;35(8):1504–24.
Vrahnas C, et al. Increased autophagy in EphrinB2-deficient osteocytes is associated with elevated secondary mineralization and brittle bone. Nat Commun. 2019;10(1):3436 This interesting study points to a potential role for autophagy in regulating secondary mineralization.
Zahm AM, Bucaro MA, Srinivas V, Shapiro IM, Adams CS. Oxygen tension regulates preosteocyte maturation and mineralization. Bone. 2008;43(1):25–31.
Takeno A, Kanazawa I, Notsu M, Tanaka KI, Sugimoto T. Glucose uptake inhibition decreases expressions of receptor activator of nuclear factor-kappa B ligand (RANKL) and osteocalcin in osteocytic MLO-Y4-A2 cells. Am J Physiol Endocrinol Metab. 2018;314(2):E115–23.
Sun N, Uda Y, Azab E, Kochen A, Santos RNCE, Shi C, et al. Effects of histone deacetylase inhibitor Scriptaid and parathyroid hormone on osteocyte functions and metabolism. J Biol Chem. 2019;294(25):9722–33.
Fidler TP, Campbell RA, Funari T, Dunne N, Balderas Angeles E, Middleton EA, et al. Deletion of GLUT1 and GLUT3 reveals multiple roles for glucose metabolism in platelet and megakaryocyte function. Cell Rep. 2017;21(6):1705.
Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–91.
Lee S-Y, Abel ED, Long F. Glucose metabolism induced by bmp signaling is essential for murine skeletal development. Nat Commun. 2018;9(1):4831.
Li, B., et al., Both aerobic glycolysis and mitochondrial respiration are required for osteoclast differentiation. Faseb j, 2020.
Esen E, Lee SY, Wice BM, Long F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30(11):1959–68.
Liu Z, et al. Mitochondrial function is compromised in cortical bone osteocytes of long-lived growth hormone receptor null mice. J Bone Miner Res. 2019;34(1):106–22 One of the first published reports studying energetics in osteocytes.
Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015;461(2):193–9.
Zhang C, et al. FOXO1 mediates advanced glycation end products induced mouse osteocyte-like MLO-Y4 cell apoptosis and dysfunctions. J Diabetes Res. 2019;2019:6757428.
Sanchez-de-Diego C, et al. Glucose restriction promotes osteocyte specification by activating a PGC-1alpha-dependent transcriptional program. iScience. 2019;15:79–94.
Maridas DE, Rendina-Ruedy E, Helderman RC, DeMambro VE, Brooks D, Guntur AR, et al. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. FASEB J. 2019;33(2):2885–98.
Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–4.
Maycas M, McAndrews KA, Sato AY, Pellegrini GG, Brown DM, Allen MR, et al. PTHrP-derived peptides restore bone mass and strength in diabetic mice: additive effect of mechanical loading. J Bone Miner Res. 2017;32(3):486–97.
Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73.
Kobayashi K, Nojiri H, Saita Y, Morikawa D, Ozawa Y, Watanabe K, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci Rep. 2015;5:9148.
Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jähn K, Yi J, et al. Beta-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018;22(6):1531–44.
Boivin G, Anthoine-Terrier C, Obrant KJ. Transmission electron microscopy of bone tissue. A review Acta Orthop Scand. 1990;61(2):170–80.
Gao J, et al. Endoplasmic reticulum mediates mitochondrial transfer within the osteocyte dendritic network. Sci Adv. 2019;5(11):eaaw7215.
Lee AR, Moon DK, Siregar A, Moon SY, Jeon RH, Son YB, et al. Involvement of mitochondrial biogenesis during the differentiation of human periosteum-derived mesenchymal stem cells into adipocytes, chondrocytes and osteocytes. Arch Pharm Res. 2019;42(12):1052–62.
Shum LC, White NS, Nadtochiy SM, Bentley KLM, Brookes PS, Jonason JH, et al. Cyclophilin D knock-out mice show enhanced resistance to osteoporosis and to metabolic changes observed in aging bone. PLoS One. 2016;11(5):e0155709.
Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73.
Guntur AR, Gerencser AA, le PT, DeMambro VE, Bornstein SA, Mookerjee SA, et al. Osteoblast-like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte-like 3T3-L1 cells prefer oxidative phosphorylation. J Bone Miner Res. 2018;33(6):1052–65.
Lee WC, Ji X, Nissim I, Long F. Malic enzyme couples mitochondria with aerobic glycolysis in osteoblasts. Cell Rep. 2020;32(10):108108.
Wang Y, Liu W, Masuyama R, Fukuyama R, Ito M, Zhang Q, et al. Pyruvate dehydrogenase kinase 4 induces bone loss at unloading by promoting osteoclastogenesis. Bone. 2012;50(1):409–19.
Vanderperre B, Herzig S, Krznar P, Hörl M, Ammar Z, Montessuit S, et al. Embryonic lethality of mitochondrial pyruvate carrier 1 deficient mouse can be rescued by a ketogenic diet. PLoS Genet. 2016;12(5):e1006056.
Vigueira PA, McCommis KS, Schweitzer GG, Remedi MS, Chambers KT, Fu X, et al. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Rep. 2014;7(6):2042–53.
Alekos NS, Moorer MC, Riddle RC. Dual effects of lipid metabolism on osteoblast function. Front Endocrinol (Lausanne). 2020;11:578194.
Sekar S, Shafie SR, Prasadam I, Crawford R, Panchal SK, Brown L, et al. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci Rep. 2017;7:46457.
Al Saedi A, et al. Mechanisms of palmitate-induced lipotoxicity in osteocytes. Bone. 2019;127:353–9.
Kim SP, et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight. 2017;2(16).
Shams-White MM, Chung M, du M, Fu Z, Insogna KL, Karlsen MC, et al. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr. 2017;105(6):1528–43.
Rizzoli R, Stevenson JC, Bauer JM, van Loon L, Walrand S, Kanis JA, et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of osteoporosis and osteoarthritis (ESCEO). Maturitas. 2014;79(1):122–32.
Rouy E, Vico L, Laroche N, Benoit V, Rousseau B, Blachier F, et al. Protein quality affects bone status during moderate protein restriction in growing mice. Bone. 2014;59:7–13.
Stegen S, Rinaldi G, Loopmans S, Stockmans I, Moermans K, Thienpont B, et al. Glutamine metabolism controls chondrocyte identity and function. Dev Cell. 2020;53(5):530–44 e8.
Yu Y, Newman H, Shen L, Sharma D, Hu G, Mirando AJ, et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 2019;29(4):966–78 e4.
Shen L, et al. Biphasic regulation of glutamine consumption by WNT during osteoblast differentiation. J Cell Sci. 2021;134(1).
Prideaux M, Kitase Y, Kimble M, O'Connell TM, Bonewald LF. Taurine, an osteocyte metabolite, protects against oxidative stress-induced cell death and decreases inhibitors of the Wnt/beta-catenin signaling pathway. Bone. 2020;137:115374.
Yang YH, Li B, Zheng XF, Chen JW, Chen K, Jiang SD, et al. Oxidative damage to osteoblasts can be alleviated by early autophagy through the endoplasmic reticulum stress pathway--implications for the treatment of osteoporosis. Free Radic Biol Med. 2014;77:10–20.
Li H, Li D, Ma Z, Qian Z, Kang X, Jin X, et al. Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy. 2018;14(10):1726–41.
Xi G, Rosen CJ, Clemmons DR. IGF-I and IGFBP-2 stimulate AMPK activation and autophagy, which are required for osteoblast differentiation. Endocrinology. 2016;157(1):268–81.
Nollet M, Santucci-Darmanin S, Breuil V, al-Sahlanee R, Cros C, Topi M, et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10(11):1965–77.
Piemontese M, Onal M, Xiong J, Han L, Thostenson JD, Almeida M, et al. Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci Rep. 2016;6:24262.
Onal M, Piemontese M, Xiong J, Wang Y, Han L, Ye S, et al. Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem. 2013;288(24):17432–40.
Zhang B, Hou R, Zou Z, Luo T, Zhang Y, Wang L, et al. Mechanically induced autophagy is associated with ATP metabolism and cellular viability in osteocytes in vitro. Redox Biol. 2018;14:492–8.
Zhu L, Chen J, Zhang J, Guo C, Fan W, Wang YM, et al. Parathyroid hormone (PTH) induces autophagy to protect osteocyte cell survival from dexamethasone damage. Med Sci Monit. 2017;23:4034–40.
Pei DD, Sun JL, Zhu CH, Tian FC, Jiao K, Anderson MR, et al. Contribution of mitophagy to cell-mediated mineralization: revisiting a 50-year-old conundrum. Adv Sci (Weinh). 2018;5(10):1800873.
Iwayama T, et al. Osteoblastic lysosome plays a central role in mineralization. Sci Adv. 2019;5(7):eaax0672.
Wang K, et al. A novel osteogenic cell line that differentiates into GFP-tagged osteocytes and forms mineral with a bone-like lacunocanalicular structure. J Bone Miner Res. 2019;34(6):979–95 This study provides a new cell model to test some of the bioenergtic pathways.
Tokarz D, Cisek R, Wein MN, Turcotte R, Haase C, Yeh SCA, et al. Intravital imaging of osteocytes in mouse calvaria using third harmonic generation microscopy. PLoS One. 2017;12(10):e0186846.
Genthial R, Beaurepaire E, Schanne-Klein MC, Peyrin F, Farlay D, Olivier C, et al. Label-free imaging of bone multiscale porosity and interfaces using third-harmonic generation microscopy. Sci Rep. 2017;7(1):3419.
Acknowledgements
We would like to apologize to all the authors whose work could not be cited due to space constraints.
Funding
This work was funded by NIGMS to A.R.G. through P20GM121301, Phase I: Mesenchymal and Neural Regulation of Metabolic Networks, Lucy Liaw, PhD, Program Director and start up research fund from MMCRI to A.R.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteocytes
Rights and permissions
About this article
Cite this article
Karthik, V., Guntur, A.R. Energy Metabolism of Osteocytes. Curr Osteoporos Rep 19, 444–451 (2021). https://doi.org/10.1007/s11914-021-00688-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-021-00688-6