Abstract
Purpose of Review
Osteocytes comprise > 95% of the cellular component in bone tissue and produce a wide range of cytokines and cellular signaling molecules in response to mechanical stimuli. In this review, we aimed to summarize the molecular mechanisms involved in the osteocyte-mediated translation of mechanical stimuli to cellular signaling, and discuss their role in skeletal (bone) diseases and extra-skeletal (non-bone) clinical complications.
Recent Findings
Two decades before, osteocytes were assumed as a dormant cells buried in bone matrix. In recent years, emerging evidences have shown that osteocytes are pivotal not only for bone homeostasis but also for vital organ functions such as muscle, kidney, and heart. Osteocyte mechanotransduction regulates osteoblast and osteoclast function and maintains bone homeostasis. Mechanical stimuli modulate the release of osteocyte-derived cytokines, signaling molecules, and extracellular cellular vesicles that regulate not only the surrounding bone cell function and bone homeostasis but also the distant organ function in a paracrine and endocrine fashion.
Summary
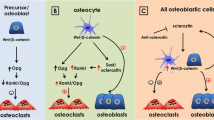
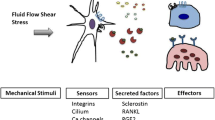
Mechanical loading and unloading modulate the osteocytic release of NO, PGE2, and ATPs that regulates multiple cellular signaling such as Wnt/β-catenin, RANKL/OPG, BMPs, PTH, IGF1, VEGF, sclerostin, and others. Therefore, the in-depth study of the molecular mechanism of osteocyte mechanotransduction could unravel therapeutic targets for various bone and non-bone-related clinical complications such as osteoporosis, sarcopenia, and cancer metastasis to bone.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cowin SC. On mechanosensation in bone under microgravity. Bone. 1998;22(5 Suppl):119S–25S.
Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13(10):1594–601.
Vico L, Hargens A. Skeletal changes during and after spaceflight. Nat Rev Rheumatol. 2018;14(4):229–45.
•• Grimm D, Grosse J, Wehland M, Mann V, Reseland JE, Sundaresan A, et al. The impact of microgravity on bone in humans. Bone. 2016;87:44–56. Reviewed the effect of micro-gravity on bone loss in human and experimental animals. Space flight-induced bone loss can be rescued in some extent by physical exercise and other interventions such as diet and medications.
Pathak JL, Bravenboer N, Luyten FP, Verschueren P, Lems WF, Klein-Nulend J, et al. Mechanical loading reduces inflammation-induced human osteocyte-to-osteoclast communication. Calcif Tissue Int. 2015;97(2):169–78.
Fahlgren A, Bratengeier C, Semeins CM, Klein-Nulend J, Bakker AD. Supraphysiological loading induces osteocyte-mediated osteoclastogenesis in a novel in vitro model for bone implant loosening. J Orthop Res. 2018;36(5):1425–34.
Delgado-Calle J, Bellido T. Osteocytes and skeletal pathophysiology. Curr Mol Biol Rep. 2015;1(4):157–67.
Delgado-Calle J, Tu X, Pacheco-Costa R, McAndrews K, Edwards R, Pellegrini GG, et al. Control of bone anabolism in response to mechanical loading and PTH by distinct mechanisms downstream of the PTH receptor. J Bone Miner Res. 2017;32(3):522–35.
• Zhou M, Li S, Pathak JL. Pro-inflammatory cytokines and osteocytes. Curr Osteoporos Rep. 2019;17(3):97–104. Reviewed the role of inflammatory cytokines in osteocytes survival and function. Inflammatory cytokines upregulate osteocyte-derived expression of sclerostin, RANKL, TNFα, FGF23, DKK1, and other signaling molecules thereby cause bone and non-bone related complications.
Wang P, Tang C, Wu J, Yang Y, Yan Z, Liu X, et al. Pulsed electromagnetic fields regulate osteocyte apoptosis, RANKL/OPG expression, and its control of osteoclastogenesis depending on the presence of primary cilia. J Cell Physiol. 2019;234(7):10588–601.
O'Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54(2):258–63.
Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, et al. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc Natl Acad Sci U S A. 2015;112(5):E478–86.
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50(1):209–17.
van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16(3):319–27.
Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet. 2003;63(3):192–7.
Van Buchem FS, Hadders HN, Ubbens R. An uncommon familial systemic disease of the skeleton: hyperostosis corticalis generalisata familiaris. Acta Radiol. 1955;44(2):109–20.
Van Hul W, Balemans W, Van Hul E, Dikkers FG, Obee H, Stokroos RJ, et al. Van Buchem disease (hyperostosis corticalis generalisata) maps to chromosome 17q12-q21. Am J Hum Genet. 1998;62(2):391–9.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38.
Fujii O, Tatsumi S, Ogata M, Arakaki T, Sakaguchi H, Nomura K, et al. Effect of osteocyte-ablation on inorganic phosphate metabolism: analysis of bone-kidney-gut axis. Front Endocrinol (Lausanne). 2017;8:359.
Tresguerres FGF, Torres J, Lopez-Quiles J, Hernandez G, Vega JA, Tresguerres IF. The osteocyte: a multifunctional cell within the bone. Ann Anat. 2020;227:151422.
Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. 2003;285(1):E1–9.
Dussold C, Gerber C, White S, Wang X, Qi L, Francis C, et al. DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res. 2019;7:12.
Martin A. Bone and heart health in chronic kidney disease: role of dentin matrix protein 1. Curr Opin Nephrol Hypertens. 2019;28(4):297–303.
Zhang BB, Hou RT, Zou Z, Luo TT, Zhang Y, Wang LY, et al. Mechanically induced autophagy is associated with ATP metabolism and cellular viability in osteocytes in vitro. Redox Biol. 2018;14:492–8.
•• Thi MM, Suadicani SO, Schaffler MB, Weinbaum S, Spray DC. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require alphaVbeta3 integrin. Proc Natl Acad Sci U S A. 2013;110(52):21012–7. Demonstrated osteocyte cell processes but not the cell bodies are mechanoresponsive. The αVβ3 integrin plays a key role in osteocyte-polarized mechanosensing and mechanotransduction.
Martin RB. Toward a unifying theory of bone remodeling. Bone. 2000;26(1):1–6.
Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–75.
Zhang D, Zhou C, Wang Q, Cai L, Du W, Li X, et al. Extracellular matrix elasticity regulates osteocyte gap junction elongation: involvement of paxillin in intracellular signal transduction. Cell Physiol Biochem. 2018;51(3):1013–26.
Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9(5):441–5.
Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104(33):13325–30.
Ponik SM, Triplett JW, Pavalko FM. Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J Cell Biochem. 2007;100(3):794–807.
Hung CT, Pollack SR, Reilly TM, Brighton CT. Real-time calcium response of cultured bone cells to fluid flow. Clin Orthop Relat Res. 1995;313:256–69.
Reich KM, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Phys. 1991;261(3 Pt 1):C428–32.
Lu XL, Huo B, Park M, Guo XE. Calcium response in osteocytic networks under steady and oscillatory fluid flow. Bone. 2012;51(3):466–73.
Jing D, Baik AD, Lu XL, Zhou B, Lai XH, Wang LY, et al. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 2014;28(4):1582–92.
Hu M, Tian GW, Gibbons DE, Jiao J, Qin YX. Dynamic fluid flow induced mechanobiological modulation of in situ osteocyte calcium oscillations. Arch Biochem Biophys. 2015;579:55–61.
Adachi T, Aonuma Y, Ito S, Tanaka M, Hojo M, Takano-Yamamoto T, et al. Osteocyte calcium signaling response to bone matrix deformation. J Biomech. 2009;42(15):2507–12.
•• Lewis KJ, Frikha-Benayed D, Louie J, Stephen S, Spray DC, Thi MM, et al. Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. Proc Natl Acad Sci USA. 2017;114(44):11775–80. Reported a mouse model with an osteocyte-targeted genetically encoded Ca2+indicator to study Ca2+ signaling in osteocytes in their authentic in vivo environment. Ca2+signaling of osteocytes in vivo, both individually and as networks, responds to mechanical loading.
Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J Bone Miner Res. 2012;27(3):563–74.
Hung CT, Allen FD, Pollack SR, Brighton CT. Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J Biomech. 1996;29(11):1411–7.
Chen X, Guo J, Yuan Y, Sun Z, Chen B, Tong X, et al. Cyclic compression stimulates osteoblast differentiation via activation of the Wnt/beta-catenin signaling pathway. Mol Med Rep. 2017;15(5):2890–6.
Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, et al. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner Metab. 2006;24(6):498–504.
Cabahug-Zuckerman P, Stout RF Jr, Majeska RJ, Thi MM, Spray DC, Weinbaum S, et al. Potential role for a specialized beta3 integrin-based structure on osteocyte processes in bone mechanosensation. J Orthop Res. 2018;36(2):642–52.
Brown GN, Leong PL, Guo XE. T-type voltage-sensitive calcium channels mediate mechanically-induced intracellular calcium oscillations in osteocytes by regulating endoplasmic reticulum calcium dynamics. Bone. 2016;88:56–63.
•• Morrell AE, Brown GN, Robinson ST, Sattler RL, Baik AD, Zhen G, et al. Mechanically induced Ca(2+) oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Res. 2018;6:6. Revealed mechanical stimulation of osteocyte-mediated activation of Ca2+-dependent contraction enhances the production and release of EVs containing bone regulatory proteins. Blocking Ca2+signaling significantly attenuates adaptation to mechanical loading in vivo, suggesting a critical role for Ca2+-mediated signaling in bone adaptation.
•• Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci USA. 2010;107(31):13648–53. Reported the importance of glycocalyx of the osteocyte dendritic process in forming strong integrin attachments that probably serve as the mechanotransducers and transmit the mechanical signals to the cell body leading to the opening of hemichannels, which permits rapid exchange of signaling factors required for bone remodeling.
Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212(1):207–14.
•• Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31(7):1075–81. Illustrated the role of osteocytic Cx43 in mechanotransduction-mediated anabolic response to the bone using osteocyte specific Cx43 knockout mice model.
Cresswell EN, Nguyen TM, Horsfield MW, Alepuz AJ, Metzger TA, Niebur GL, et al. Mechanically induced bone formation is not sensitive to local osteocyte density in rat vertebral cancellous bone. J Orthop Res. 2018;36(2):672–81.
Zhang JN, Zhao Y, Liu C, Han ES, Yu X, Lidington D, et al. The role of the sphingosine-1-phosphate signaling pathway in osteocyte mechanotransduction. Bone. 2015;79:71–8.
Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res. 1999;14(7):1123–31.
Vatsa A, Smit TH, Klein-Nulend J. Extracellular NO signalling from a mechanically stimulated osteocyte. J Biomech. 2007;40:S89–95.
Vatsa A, Mizuno D, Smit TH, Schmidt CF, MacKintosh FC, Klein-Nulend J. Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J Bone Miner Res. 2006;21(11):1722–8.
Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Phys. 1999;276(1):E171–8.
Lean JM, Jagger CJ, Chambers TJ, Chow JW. Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Phys. 1995;268(2 Pt 1):E318–27.
Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone. 2010;47(5):872–81.
Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400.
Hua K, Ferland RJ. Primary cilia proteins: ciliary and extraciliary sites and functions. Cell Mol Life Sci. 2018;75(9):1521–40.
Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19(13):R526–R35.
Yuan X, Serra RA, Yang SY. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann N Y Acad Sci. 2015;1335:78–99.
•• Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, et al. Primary cilia are not calcium-responsive mechanosensors. Nature. 2016;531(7596):656–60. Kidney cells with calcium indicator encoded primary cilia revealed that primary cilia-mediated mechanosensation is not via cilia-specific Ca(2+) influxes.
Delaine-Smith RM, Sittichokechaiwut A, Reilly GC. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB J. 2014;28(1):430–9.
Xiao Z, Dallas M, Qiu N, Nicolella D, Cao L, Johnson M, et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011;25(7):2418–32.
Xiao ZS, Zhang SQ, Cao L, Qiu N, David V, Quarles LD. Conditional disruption of Pkd1 in osteoblasts results in osteopenia due to direct impairment of bone formation. J Biol Chem. 2010;285(2):1177–87.
Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. FASEB J. 2016;30(4):1504–11.
Qiu N, Xiao Z, Cao L, Buechel MM, David V, Roan E, et al. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J Cell Sci. 2012;125(Pt 8):1945–57.
Temiyasathit S, Tang WJ, Leucht P, Anderson CT, Monica SD, Castillo AB, et al. Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PLoS One. 2012;7(3):e33368.
Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275–302.
Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2(11):793–805.
Goldmann WH. Role of vinculin in cellular mechanotransduction. Cell Biol Int. 2016;40(3):241–56.
Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32(Pt3):416–20.
Kulkarni RN, Bakker AD, Gruber EV, Chae TD, Veldkamp JBB, Klein-Nulend J, et al. MT1-MMP modulates the mechanosensitivity of osteocytes. Biochem Biophys Res Commun. 2012;417(2):824–9.
Bell S, Terentjev EM. Focal adhesion kinase: the reversible molecular mechanosensor. Biophys J. 2017;112(11):2439–50.
Santos A, Bakker AD, Zandieh-Doulabi B, de Blieck-Hogervorst JMA, Klein-Nulend J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem Biophys Res Commun. 2010;391(1):364–9.
Young SRL, Gerard-O'Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24:411–24.
Santos A, Bakker AD, Willems HM, Bravenboer N, Bronckers AL, Klein-Nulend J. Mechanical loading stimulates BMP7, but not BMP2, production by osteocytes. Calcif Tissue Int. 2011;89(4):318–26.
Sun Z, Lambacher A, Fassler R. Nascent adhesions: from fluctuations to a hierarchical organization. Curr Biol. 2014;24(17):R801–3.
Kaneko K, Ito M, Naoe Y, Lacy-Hulbert A, Ikeda K. Integrin alphav in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun. 2014;447(2):352–7.
Geoghegan IP, Hoey DA, McNamara LM. Integrins in osteocyte biology and mechanotransduction. Curr Osteoporos Rep. 2019;17(4):195–206.
Geoghegan IP, Hoey DA, McNamara LM. Estrogen deficiency impairs integrin alphavbeta3-mediated mechanosensation by osteocytes and alters osteoclastogenic paracrine signalling. Sci Rep. 2019;9(1):4654.
McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec (Hoboken). 2009;292(3):355–63.
Bidwell JP, Pavalko FM. Mechanosomes carry a loaded message. Sci Signal. 2010;3(153):pe51.
•• Sato AY, Li T, Robling AG. FAK expression in osteocytes is dispensable for bone accrual and for the anabolic response of cortical and cancellous bone to mechanical loading in female mice. J Bone Miner Res. 2018;32. Avialable at https://www.asbmr.org/education/AbstractDetail?aid=048482c3-d377-4a4a-98a5-20c68712a5b3. The first time illustrated that the osteocytic FAK expression in depensible for mechanical loading-mediated anabolic effect on bone in vivo using FAKΔOTand FAKf/fmice. This finding is contrary to previous reports form the in vitro studies.
Fox SW, Chambers TJ, Chow JW. Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Phys. 1996;270(6 Pt 1):E955–60.
Turner CH, Takano Y, Owan I, Murrell GA. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am J Phys. 1996;270(4 Pt 1):E634–9.
•• Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4. Reported severe osteopetrotic phenotype in osteocyte-specific RANKL knockout mice, indicating osteocytes as a major source of RANKL in bone remodelingin vivo.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–41.
Bakker AD, Silva VC, Krishnan R, Bacabac RG, Blaauboer ME, Lin YC, et al. Tumor necrosis factor alpha and interleukin-1beta modulate calcium and nitric oxide signaling in mechanically stimulated osteocytes. Arthritis Rheum. 2009;60(11):3336–45.
Delgado-Calle J, Riancho JA, Klein-Nulend J. Nitric oxide is involved in the down-regulation of SOST expression induced by mechanical loading. Calcif Tissue Int. 2014;94(4):414–22.
Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271(52):33157–60.
Bakker AD, Klein-Nulend J, Burger EH. Mechanotransduction in bone cells proceeds via activation of COX-2, but not COX-1. Biochem Biophys Res Commun. 2003;305(3):677–83.
Joldersma M, Burger EH, Semeins CM, Klein-Nulend J. Mechanical stress induces COX-2 mRNA expression in bone cells from elderly women. J Biomech. 2000;33(1):53–61.
Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16(7):3100–6.
Gupta A, Chatree S, Buo AM, Moorer MC, Stains JP. Connexin43 enhances Wnt and PGE2-dependent activation of beta-catenin in osteoblasts. Pflugers Arch. 2019;471(9):1235–43.
Kringelbach TM, Aslan D, Novak I, Ellegaard M, Syberg S, Andersen CK, et al. Fine-tuned ATP signals are acute mediators in osteocyte mechanotransduction. Cell Signal. 2015;27(12):2401–9.
•• Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–91 Indicated the anabolic role of activated osteocytic LRP5 signaling in bone mass, suggesting it as a possible approach to treat osteoporosis.
Bullock WA, Pavalko FM, Robling AG. Osteocytes and mechanical loading: the Wnt connection. Orthod Craniofacial Res. 2019;22(Suppl 1):175–9.
• Lara-Castillo N, Kim-Weroha NA, Kamel MA, Javaheri B, Ellies DL, Krumlauf RE, et al. In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone. 2015;76:58–66. Reported the rapid activation of β-catenin in vitro and in vivo in response to mechanical loading of osteocytes is via the early release of prostaglandin.
Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–711.
Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V high bone mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone. 2011;49(2):184–93.
Zhao L, Shim JW, Dodge TR, Robling AG, Yokota H. Inactivation of Lrp5 in osteocytes reduces young's modulus and responsiveness to the mechanical loading. Bone. 2013;54(1):35–43.
Kogawa M, Khalid KA, Wijenayaka AR, Ormsby RT, Evdokiou A, Anderson PH, et al. Recombinant sclerostin antagonizes effects of ex vivo mechanical loading in trabecular bone and increases osteocyte lacunar size. Am J Phys Cell Phys. 2018;314(1):C53–61.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–75.
Morse A, McDonald MM, Kelly NH, Melville KM, Schindeler A, Kramer I, et al. Mechanical load increases in bone formation via a sclerostin-independent pathway. J Bone Miner Res. 2014;29(11):2456–67.
Reid IR. Targeting sclerostin in postmenopausal osteoporosis: focus on romosozumab and blosozumab. BioDrugs. 2017;31(4):289–97.
Saliba W, El-Haddad B. Secondary hyperparathyroidism: pathophysiology and treatment. J Am Board Fam Med. 2009;22(5):574–81.
Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, et al. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142(9):4047–54.
Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434–46.
Falk B, Haddad F, Klentrou P, Ward W, Kish K, Mezil Y, et al. Differential sclerostin and parathyroid hormone response to exercise in boys and men. Osteoporos Int. 2016;27(3):1245–9.
Gardinier JD, Al-Omaishi S, Morris MD, Kohn DH. PTH signaling mediates perilacunar remodeling during exercise. Matrix Biol. 2016;52–54:162–75.
Maycas M, McAndrews KA, Sato AY, Pellegrini GG, Brown DM, Allen MR, et al. PTHrP-derived peptides restore bone mass and strength in diabetic mice: additive effect of mechanical loading. J Bone Miner Res. 2017;32(3):486–97.
Camirand A, Goltzman D, Gupta A, Kaouass M, Panda D, Karaplis A. The role of parathyroid hormone-related protein (PTHrP) in osteoblast response to microgravity: mechanistic implications for osteoporosis development. PLoS One. 2016;11(7):e0160034.
•• Maycas M, Ardura JA, de Castro LF, Bravo B, Gortazar AR, Esbrit P. Role of the parathyroid hormone type 1 receptor (PTH1R) as a mechanosensor in osteocyte survival. J Bone Miner Res. 2015;30(7):1231–44. Reported PTH1R as an important component of mechanical signal transduction in osteocytes. PTH1R activation by PTHrP-independent and PTHrP-dependent mechanisms is a key event for the prosurvival action induced by mechanical stimulation in osteocytes.
Kurata K, Heino TJ, Higaki H, Vaananen HK. Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J Bone Miner Res. 2006;21(4):616–25.
Xiong J, O'Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res. 2012;27(3):499–505.
• Spatz JM, Wein MN, Gooi JH, Qu YL, Garr JL, Liu S, et al. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J Biol Chem. 2015;290(27):16744–58. Showed mechanical unloading of osteocytes in vitro as a upregulator of SOST/sclerostin and RANKL/OPG ratio. This result highly supports the findings from in vivo mechanical unloading studies and suggest osteocyte targeted therapy as a potential approach to treat osteoporosis and disuse-induced bone loss.
You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42(1):172–9.
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3(11):1009–13.
Mohan S, Baylink DJ. Impaired skeletal growth in mice with haploinsufficiency of IGF-I: genetic evidence that differences in IGF-I expression could contribute to peak bone mineral density differences. J Endocrinol. 2005;185(3):415–20.
Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, et al. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16(12):2320–9.
Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21(9):1350–8.
Reijnders CM, Bravenboer N, Tromp AM, Blankenstein MA, Lips P. Effect of mechanical loading on insulin-like growth factor-I gene expression in rat tibia. J Endocrinol. 2007;192(1):131–40.
Lau KHW, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li ZH, et al. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Phys Endocrinol Metab. 2013;305(2):E271–E81.
Saini V, Marengi DA, Barry KJ, Fulzele KS, Heiden E, Liu X, et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288(28):20122–34.
Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J Bone Miner Res. 2011;26(5):1035–46.
Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002;17(3):493–501.
Kesavan C, Wergedal JE, Lau KH, Mohan S. Conditional disruption of IGF-I gene in type 1alpha collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. Am J Physiol Endocrinol Metab. 2011;301(6):E1191–7.
Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res. 2004;19(3):436–46.
Kubota T, Elalieh HZ, Saless N, Fong C, Wang YM, Babey M, et al. Insulin-like growth factor-1 receptor in mature osteoblasts is required for periosteal bone formation induced by reloading. Acta Astronaut. 2013;92(1):73–8.
Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts). J Tissue Eng Regen Med. 2008;2(1):1–13.
Wang Z, Guo J. Mechanical induction of BMP-7 in osteocyte blocks glucocorticoid-induced apoptosis through PI3K/AKT/GSK3beta pathway. Cell Biochem Biophys. 2013;67(2):567–74.
Kopf J, Petersen A, Duda GN, Knaus P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biol. 2012;10:37.
Schwarz C, Wulsten D, Ellinghaus A, Lienau J, Willie BM, Duda GN. Mechanical load modulates the stimulatory effect of BMP2 in a rat nonunion model. Tissue Eng Part A. 2013;19(1–2):247–54.
Lai CF, Cheng SL. Alphavbeta integrins play an essential role in BMP-2 induction of osteoblast differentiation. J Bone Miner Res. 2005;20(2):330–40.
Holguin N, Brodt MD, Silva MJ. Activation of Wnt signaling by mechanical loading is impaired in the bone of old mice. J Bone Miner Res. 2016;31(12):2215–26.
Bakker AD, Kulkarni RN, Klein-Nulend J, Lems WF. IL-6 alters osteocyte signaling toward osteoblasts but not osteoclasts. J Dent Res. 2014;93(4):394–9.
Sanchez C, Gabay O, Salvat C, Henrotin YE, Berenbaum F. Mechanical loading highly increases IL-6 production and decreases OPG expression by osteoblasts. Osteoarthr Cartil. 2009;17(4):473–81.
Hao ZC, Ma YY, Wu J, Li XX, Chen HL, Shen JF, et al. Osteocytes regulate osteoblast differentiation and osteoclast activity through Interleukin-6 under mechanical loading. RSC Adv. 2017;7(79):50200–9.
Metzger CE, Brezicha JE, Elizondo JP, Narayanan SA, Hogan HA, Bloomfield SA. Differential responses of mechanosensitive osteocyte proteins in fore- and hindlimbs of hindlimb-unloaded rats. Bone. 2017;105:26–34. https://doi.org/10.1016/j.bone.2017.08.002.
Kaneshiro S, Ebina K, Shi K, Higuchi C, Hirao M, Okamoto M, et al. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab. 2014;32(4):378–92.
Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126(2):509–26.
Liu C, Zhang X, Wu M, You L. Mechanical loading up-regulates early remodeling signals from osteocytes subjected to physical damage. J Biomech. 2015;48(16):4221–8.
Dumas V, Perrier A, Malaval L, Laroche N, Guignandon A, Vico L, et al. The effect of dual frequency cyclic compression on matrix deposition by osteoblast-like cells grown in 3D scaffolds and on modulation of VEGF variant expression. Biomaterials. 2009;30(19):3279–88.
de Castro LF, Maycas M, Bravo B, Esbrit P, Gortazar A. VEGF receptor 2 (VEGFR2) activation is essential for osteocyte survival induced by mechanotransduction. J Cell Physiol. 2015;230(2):278–85.
Yuan Y, Zhang LL, Tong XY, Zhang M, Zhao YL, Guo JM, et al. Mechanical stress regulates bone metabolism through microRNAs. J Cell Physiol. 2017;232(6):1239–45.
Iwawaki Y, Mizusawa N, Iwata T, Higaki N, Goto T, Watanabe M, et al. MiR-494-3p induced by compressive force inhibits cell proliferation in MC3T3-E1 cells. J Biosci Bioeng. 2015;120(4):456–62.
•• Zuo B, Zhu J, Li J, Wang C, Zhao X, Cai G, et al. microRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. J Bone Miner Res. 2015;30(2):330–45. Reported mechanosensitive miR103a as a negative regulator of osteogenesis. Mechanical unloading enhances expression of miR103a in vitro and in vivo. Pretreatment with antagomir-103a partly rescues the osteoporosis caused by mechanical unloading.
Zeng Q, Wang Y, Gao J, Yan Z, Li Z, Zou X, et al. miR-29b-3p regulated osteoblast differentiation via regulating IGF-1 secretion of mechanically stimulated osteocytes. Cell Mol Biol Lett. 2019;24:11.
Hemmatian H, Bakker AD, Klein-Nulend J, van Lenthe GH. Aging, osteocytes, and mechanotransduction. Curr Osteoporos Rep. 2017;15(5):401–11.
Sapir-Koren R, Livshits G. Is interaction between age-dependent decline in mechanical stimulation and osteocyte-estrogen receptor levels the culprit for postmenopausal-impaired bone formation? Osteoporos Int. 2013;24(6):1771–89.
Aguirre JI, Plotkin LI, Gortazar AR, Millan MM, O'Brien CA, Manolagas SC, et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem. 2007;282(35):25501–8.
Lee KC, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol. 2004;182(2):193–201.
Sugiyama T, Galea GL, Lanyon LE, Price JS. Mechanical loading-related bone gain is enhanced by tamoxifen but unaffected by fulvestrant in female mice. Endocrinology. 2010;151(12):5582–90.
Bakker AD, Klein-Nulend J, Tanck E, Heyligers IC, Albers GH, Lips P, et al. Different responsiveness to mechanical stress of bone cells from osteoporotic versus osteoarthritic donors. Osteoporos Int. 2006;17(6):827–33.
Sankardas PA, Lavu V, Lakakula B, Rao SR. Differential expression of periostin, sclerostin, receptor activator of nuclear factor-kappaB, and receptor activator of nuclear factor-kappaB ligand genes in severe chronic periodontitis. J Investig Clin Dent. 2019;10(1):e12369.
Chatzopoulos GS, Mansky KC, Lunos S, Costalonga M, Wolff LF. Sclerostin and WNT-5a gingival protein levels in chronic periodontitis and health. J Periodontal Res. 2019;54(5):555–65.
McDonald MM, Delgado-Calle J. Sclerostin: an emerging target for the treatment of cancer-induced bone disease. Curr Osteoporos Rep. 2017;15(6):532–41.
Galea GL, Lanyon LE, Price JS. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone. 2017;96:38–44.
Liao HW, Huang TH, Chang YH, Liou HH, Chou YH, Sue YM et al. Exercise alleviates osteoporosis in rats with mild chronic kidney disease by decreasing sclerostin production. Int J Mol Sci. 2019;20(8).
Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 2013;28(4):865–74.
Stegen S, Stockmans I, Moermans K, Thienpont B, Maxwell PH, Carmeliet P, et al. Osteocytic oxygen sensing controls bone mass through epigenetic regulation of sclerostin. Nat Commun. 2018;9:2557.
Riddle RC, Leslie JM, Gross TS, Clemens TL. Hypoxia-inducible factor-1alpha protein negatively regulates load-induced bone formation. J Biol Chem. 2011;286(52):44449–56.
Bergstrom I, Isaksson H, Koskela A, Tuukkanen J, Ohlsson C, Andersson G, et al. Prednisolone treatment reduces the osteogenic effects of loading in mice. Bone. 2018;112:10–8.
Lionikaite V, Henning P, Drevinge C, Shah FA, Palmquist A, Wikstrom P, et al. Vitamin a decreases the anabolic bone response to mechanical loading by suppressing bone formation. FASEB J. 2019;33(4):5237–47.
Parajuli A, Liu C, Li W, Gu X, Lai X, Pei S, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015;81:152–60.
Seref-Ferlengez Z, Maung S, Schaffler MB, Spray DC, Suadicani SO, Thi MM. P2X7R-Panx1 complex impairs bone mechanosignaling under high glucose levels associated with type-1 diabetes. PLoS One. 2016;11(5):e0155107.
Tan SD, Kuijpers-Jagtman AM, Semeins CM, Bronckers AL, Maltha JC, Von den Hoff JW, et al. Fluid shear stress inhibits TNFalpha-induced osteocyte apoptosis. J Dent Res. 2006;85(10):905–9.
Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J. Mechanical loading prevents the stimulating effect of IL-1beta on osteocyte-modulated osteoclastogenesis. Biochem Biophys Res Commun. 2012;420(1):11–6.
Liao C, Cheng T, Wang S, Zhang C, Jin L, Yang Y. Shear stress inhibits IL-17A-mediated induction of osteoclastogenesis via osteocyte pathways. Bone. 2017;101:10–20.
Pathak JL, Bravenboer N, Verschueren P, Lems WF, Luyten FP, Klein-Nulend J, et al. Inflammatory factors in the circulation of patients with active rheumatoid arthritis stimulate osteoclastogenesis via endogenous cytokine production by osteoblasts. Osteoporos Int. 2014;25(10):2453–63.
•• Pathak JL, Bakker AD, Luyten FP, Verschueren P, Lems WF, Klein-Nulend J, et al. Systemic inflammation affects human osteocyte-specific protein and cytokine expression. Calcif Tissue Int. 2016;98(6):596–608. Showed the effect of exogenous recombinant pro-inflammatory cytokines and RA-serum on upregulation of signaling molecules and cytokine expression in human osteocytes cultured in ex-vivo.
Li Z, Betts D, Kuhn G, Schirmer M, Muller R, Ruffoni D. Mechanical regulation of bone formation and resorption around implants in a mouse model of osteopenic bone. J R Soc Interface. 2019;16(152):20180667.
•• Pagnotti GM, Chan ME, Adler BJ, Shroyer KR, Rubin J, Bain SD, et al. Low intensity vibration mitigates tumor progression and protects bone quantity and quality in a murine model of myeloma. Bone. 2016;90:69–79. Tested the effect of low intensity vibration (mild form of mechanical stimuli) on multiple myeloma related bone complications. Low intensity vibration mitigates the multiple myeloma disease progression and myeloma-induced osteolysis and bone loss.
Lynch ME, Brooks D, Mohanan S, Lee MJ, Polamraju P, Dent K, et al. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J Bone Miner Res. 2013;28(11):2357–67.
Wang WB, Sarazin BA, Kornilowicz G, Lynch ME. Mechanically-loaded breast cancer cells modify osteocyte mechanosensitivity by secreting factors that increase osteocyte dendrite formation and downstream resorption. Front Endocrinol. 2018;9:352.
Kaji H. Interaction between muscle and bone. J Bone Metab. 2014;21(1):29–40.
Avin KG, Bloomfield SA, Gross TS, Warden SJ. Biomechanical aspects of the muscle-bone interaction. Curr Osteoporos Rep. 2015;13(1):1–8.
Jahn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M, et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cell Mater. 2012;24:197–209.
Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jahn K, Yi J, et al. Beta-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018;22(6):1531–44.
Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/beta-catenin pathway. JBMR Plus. 2017;1(2):86–100.
Ma YV, Lam C, Dalmia S, Gao P, Young J, Middleton K, et al. Mechanical regulation of breast cancer migration and apoptosis via direct and indirect osteocyte signaling. J Cell Biochem. 2018;119(7):5665–75.
•• Ma YV, Xu L, Mei X, Middleton K, You L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J Cell Biochem. 2018;120:7590–601. Demonstrated the inhibitory effect of mechanically loaded osteocytes on breast cancer metastasis to bone through the downregulation of MMP-9 and FZD4. Mechanically loaded osteocytes reduce endothelial cells permeability and the adhesion of breast cancer cells on endothelial monolayers.
Mei X, Middleton K, Shim D, Wan Q, Xu L, Ma YV, et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr Biol (Camb). 2019;11(4):119–29.
Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006;108(13):3992–6.
Vallet S, Filzmoser JM, Pecherstorfer M, Podar K. Myeloma bone disease: update on pathogenesis and novel treatment strategies. Pharmaceutics. 2018;10(4).
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–91.
Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i90.
Gao Y, Wang J, Zhao F. CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 2015;16:4.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Qian DY, Yan GB, Bai B, Chen Y, Zhang SJ, Yao YC, et al. Differential circRNA expression profiles during the BMP2-induced osteogenic differentiation of MC3T3-E1 cells. Biomed Pharmacother. 2017;90:492–9.
Filipowska J, Tomaszewski KA, Niedzwiedzki L, Walocha JA, Niedzwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20(3):291–302.
Wei F, Xiao Y. Modulation of the osteoimmune environment in the development of biomaterials for osteogenesis. Adv Exp Med Biol. 2018;1077:69–86.
Funding
This work was supported by Project of Education of Guangdong Province, China (2017KQNCX162), Project of Guangzhou Municipal Health Commission, China (20181A011103), and the Project of Liwan District Science and Technology, Gunagzhou, China (201804015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Yongyong Yan, Liping Wang, Linhu Ge, Janak L. Pathak declare no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteocytes
Rights and permissions
About this article
Cite this article
Yan, Y., Wang, L., Ge, L. et al. Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications. Curr Osteoporos Rep 18, 67–80 (2020). https://doi.org/10.1007/s11914-020-00564-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00564-9