Abstract
Purpose of Review
We reviewed recent progress on the role of sclerostin (SOST) and its effects on the immune system in order to summarize the current state of knowledge in osteoimmunology, in regard to hematopoiesis, lymphopoiesis, and inflammation.
Recent Findings
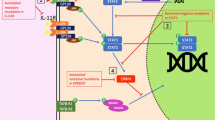
Changes in sclerostin levels affect distinct niches within the bone marrow that support hematopoietic stem cells and B cell development. Sclerostin’s regulation of adipogenesis could also be important for immune cell maintenance with age. Surprisingly, B cell development in the bone marrow is influenced by Sost produced by mesenchymal stem cells and osteoblasts, but not by osteocytes. Additionally, extramedullary hematopoiesis in the spleen and increased pro-inflammatory cytokine levels in the bone marrow are observed in global Sost−/− mice.
Summary
In addition to changes in bone marrow density, sclerostin depletion affects B lymphopoiesis and myelopoiesis, as well as other changes within the bone marrow cavity that could affect hematopoiesis. It is therefore important to monitor for hematopoietic changes in patients receiving sclerostin-depleting therapies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–9.
van Bezooijen RL, Svensson JP, Eefting D, Visser A, van der Horst G, Karperien M, et al. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res. 2007;22:19–28.
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–4.
Gori F, Lerner U, Ohlsson C, Baron R. A new WNT on the bone: WNT16, cortical bone thickness, porosity and fractures. Bonekey Rep. 2015;4:669.
Yavropoulou MP, Xygonakis C, Lolou M, Karadimou F, Yovos JG. The sclerostin story: from human genetics to the development of novel anabolic treatment for osteoporosis. Hormones (Athens). 2014;13:323–37.
McClung MR. Sclerostin antibodies in osteoporosis: latest evidence and therapeutic potential. Ther Adv Musculoskelet Dis. 2017;9:263–70.
Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–83.
Schaniel C, Sirabella D, Qiu J, Niu X, Lemischka IR, Moore KA. Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood. 2011;118:2420–9.
Richter J, Traver D, Willert K. The role of Wnt signaling in hematopoietic stem cell development. Crit Rev Biochem Mol Biol. 2017;52:414–24.
McClung MR. Romosozumab for the treatment of osteoporosis. Osteoporos Sarcopenia. 2018;4:11–5.
Markham A. Romosozumab: first global approval. Drugs. 2019;79:471–6.
Denkinger MD, Leins H, Schirmbeck R, Florian MC, Geiger H. HSC aging and senescent immune remodeling. Trends Immunol. 2015;36:815–24.
Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37.
Cain CJ, Rueda R, McLelland B, Collette NM, Loots GG, Manilay JO. Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res. 2012;27:1451–61.
Yee, C. S., J. O. Manilay, J. C. Chang, N. R. Hum, D. K. Murugesh, J. Bajwa, M. E. Mendez, A. E. Economides, D. J. Horan, A. G. Robling, and G. G. Loots. 2018. Conditional deletion of Sost in MSC-derived lineages identifies specific cell-type contributions to bone mass and B-cell development. J Bone Miner Res 33: 1748–1759. This study demonstrated thatPrx1+mesenchymal stem cells significantly contribute to the paracrine pool of sclerostin in the bone, and that conditional deletion ofSostin Prx1-expressing cells recapitulates the increased bone mass phenotype observed in the globalSost−/−mouse. Furthermore, this study demonstrated thatSostspecifically in mesenchymal stem cells, rather thanSostin osteocytes, influences B lymphocyte development.
Horowitz MC, Fretz JA. Sclerostin: a new mediator of crosstalk between the skeletal and immune systems. J Bone Miner Res. 2012;27:1448–50.
Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2011;12:49–60.
Lo Celso C, Scadden DT. The haematopoietic stem cell niche at a glance. J Cell Sci. 2011;124:3529–35.
Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–46.
Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–30.
Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–5.
Funk PE, Varas A, Witte PL. Activity of stem cell factor and IL-7 in combination on normal bone marrow B lineage cells. J Immunol. 1993;150:748–52.
Cordeiro Gomes A, Hara T, Lim VY, Herndler-Brandstetter D, Nevius E, Sugiyama T, et al. Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity. 2016;45:1219–31.
Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6:107–16.
Yu VW, Lymperi S, Oki T, Jones A, Swiatek P, Vasic R, et al. Distinctive mesenchymal-parenchymal cell pairings govern B cell differentiation in the bone marrow. Stem Cell Reports. 2016;7:220–35.
Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–73.
Aurrand-Lions M, Mancini SJC. Murine Bone marrow niches from hematopoietic stem cells to B cells. Int J Mol Sci. 2018;19.
Loots, G. G., A. G. Robling, J. C. Chang, D. K. Murugesh, J. Bajwa, C. Carlisle, J. O. Manilay, A. Wong, C. E. Yellowley, and D. C. Genetos. 2018. Vhl deficiency in osteocytes produces high bone mass and hematopoietic defects. Bone 116: 307–314. This study revealed a novel influence ofVhlin osteocytes and maintenance of bone through regulation of canonical Wnt signaling. Conditional deletion ofVhlin osteocytes using Dmp-Cre results in a high bone mass phenotype and reducedSostexpression. In addition, B cell development inVhl-conditional knockout mice is severely reduced and myelopoiesis was increased, and more extensive than the effect observed in globalSost−/−mice.
Fujiwara Y, Piemontese M, Liu Y, Thostenson JD, Xiong J, O’Brien CA. RANKL (receptor activator of NFkappaB ligand) produced by osteocytes is required for the increase in B cells and Bone loss caused by estrogen deficiency in mice. J Biol Chem. 2016;291:24838–50.
Panaroni C, Fulzele K, Saini V, Chubb R, Pajevic PD, Wu JY. PTH signaling in Osteoprogenitors is essential for B-lymphocyte differentiation and mobilization. J Bone Miner Res. 2015;30:2273–86.
Saito K. Effects of hyperoxia on phospholipid metabolism and on intracellular structure of cultured type II pneumocytes. Kokyu To Junkan. 1986;34:1079–85.
Cain CJ, Manilay JO. Hematopoietic stem cell fate decisions are regulated by Wnt antagonists: comparisons and current controversies. Exp Hematol. 2013;41:3–16.
Chen D, Li Y, Zhou Z, Wu C, Xing Y, Zou X, et al. HIF-1alpha inhibits Wnt signaling pathway by activating Sost expression in osteoblasts. PLoS One. 2013;8:e65940.
Stegen S, Stockmans I, Moermans K, Thienpont B, Maxwell PH, Carmeliet P, et al. Osteocytic oxygen sensing controls bone mass through epigenetic regulation of sclerostin. Nat Commun. 2018;9:2557.
Zuo GL, Zhang LF, Qi J, Kang H, Jia P, Chen H, et al. Activation of HIFa pathway in mature osteoblasts disrupts the integrity of the osteocyte/canalicular network. PLoS One. 2015;10:e0121266.
Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–5.
Chow, A., J. Mason, L. Coney, J. Bajwa, C. Carlisle, A. Zaslavsky, Y. Pellman, M. E. García-Ojeda, A. Economides, G. G. Loots, and J. O. Manilay. 2018. Sclerostin deficiency alters peripheral B lymphocyte responses in mice. bioRxiv: 357772.
McClung MR, Brown JP, Diez-Perez A, Resch H, Caminis J, Meisner P, et al. Effects of 24 months of treatment with Romosozumab followed by 12 months of Denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33:1397–406.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35.
Pronk CJ, Veiby OP, Bryder D, Jacobsen SE. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: involvement of two distinct receptors. J Exp Med. 2011;208:1563–70.
Kennedy, D. E., and K. L. Knight. 2017. Inflammatory changes in bone marrow microenvironment sssociated with declining B lymphopoiesis. J Immunol 198: 3471–3479. This study revealed that in rabbits, increases in myelopoiesis and bone marrow fat levels correspond with an early block of B lymphopoiesis and have been shownin vitroto inhibit B lymphopoiesis. This article also concludes that two inflammatory molecules produced by myeloid cells, IL-1β and S100A9, are increased in the BM during B lymphopoiesis arrest and inhibit B lymphopoiesisin vitro.
Chang, J. C., B. A. Christiansen, D. K. Murugesh, A. Sebastian, N. R. Hum, N. M. Collette, S. Hatsell, A. N. Economides, C. D. Blanchette, and G. G. Loots. 2018. SOST/Sclerostin improves posttraumatic osteoarthritis and inhibits MMP2/3 expression after injury. J Bone Miner Res 33: 1105–1113. Chang et al. demonstrated thatSostactivation in response to joint injury is TNFα and NF-κB dependent, and that in PTOA, SOST functions as a protective molecule to prevent cartilage degradation in subsequent traumatic injury by downregulating Wnt-dependent catabolic enzymes.
Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12.
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–7.
van Lierop AH, Hamdy NA, van Egmond ME, Bakker E, Dikkers FG, Papapoulos SE. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res. 2013;28:848–54.
MacNabb C, Patton D, Hayes JS. Sclerostin antibody therapy for the treatment of osteoporosis: clinical prospects and challenges. J Osteoporos. 2016;2016:6217286.
Horowitz MC, Berry R, Holtrup B, Sebo Z, Nelson T, Fretz JA, et al. Bone marrow adipocytes. Adipocyte. 2017;6:193–204.
Lassailly F, Foster K, Lopez-Onieva L, Currie E, Bonnet D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood. 2013;122:1730–40.
Geerman S, Hickson S, Brasser G, Pascutti MF, Nolte MA. Quantitative and qualitative analysis of bone marrow CD8(+) T cells from different bones uncovers a major contribution of the bone marrow in the vertebrae. Front Immunol. 2015;6:660.
Fairfield, H., C. Falank, E. Harris, V. Demambro, M. McDonald, J. A. Pettitt, S. T. Mohanty, P. Croucher, I. Kramer, M. Kneissel, C. J. Rosen, and M. R. Reagan. 2018. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol 233: 1156–1167. Fairfield et al. first demonstrated a direct link between sclerostin produced by osteocytes and the promotion of the development of bone marrow adipose tissue by SOSTin vitroandin vivo, in mice. This paper reveals another possible alteration of the bone marrow niche that could affect hematopoiesis and immune cell lineages when sclerostin is depleted.
Li S, Huang B, Jiang B, Gu M, Yang X, Yin Y. Sclerostin antibody mitigates estrogen deficiency-inducted marrow lipid accumulation assessed by proton MR spectroscopy. Front Endocrinol (Lausanne). 2019;10:159.
Kennedy DE, Witte PL, Knight KL. Bone marrow fat and the decline of B lymphopoiesis in rabbits. Dev Comp Immunol. 2016;58:30–9.
Turner RT, Martin SA, Iwaniec UT. Metabolic coupling between bone marrow adipose tissue and hematopoiesis. Curr Osteoporos Rep. 2018;16:95–104.
Authors’ Roles
Drafting and revising manuscript: CD and JOM; approving final version of manuscript: JOM.
Funding
This work was supported by University of California (UC), Merced faculty research funding, National Institutes of Health Award 1R15HL121786-01A1, Halcyon-Dixon Trust award to JOM, and UC Graduate Student Fellowships to CD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards and institutional approvals.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteoimmunology
Rights and permissions
About this article
Cite this article
Donham, C., Manilay, J.O. The Effects of Sclerostin on the Immune System. Curr Osteoporos Rep 18, 32–37 (2020). https://doi.org/10.1007/s11914-020-00563-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00563-w