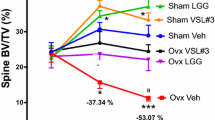
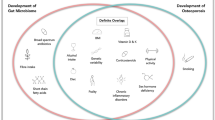
Abstract
Purpose of Review
The gut microbiota can be considered a hidden organ that plays essential roles in host homeostasis. Exploration of the effects of microbiota on bone has just begun. Complimentary studies using germ-free mice, antibiotic, and probiotic treatments reveal a complicated relationship between microbiota and bone. Here, we review recent reports addressing the effect of gut microbiota on bone health, discuss potential reasons for discrepant findings, and explore potential mechanisms for these effects.
Recent Findings
Manipulation of microbiota by colonization of germ-free mice, antibiotics, or probiotic supplementation significantly alters bone remodeling, bone development and growth, as well as bone mechanical strength. Different experimental models reveal context-dependent effects of gut microbiota on bone.
Summary
By examining phenotypic effects, experimental context, and proposed mechanisms, revealed by recent reports, we hope to provide comprehensive and fresh insights into the many facets of microbiota and bone interactions.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73. doi:10.1126/science.1223490.
Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146:1525–33. doi:10.1053/j.gastro.2014.02.008.
Flint HJ. Obesity and the gut microbiota. J Clin Gastroenterol. 2011;45(Suppl):S128–32. doi:10.1097/MCG.0b013e31821f44c4.
Charles JF, Ermann J, Aliprantis AO. The intestinal microbiome and skeletal fitness: connecting bugs and bones. Clin Immunol. 2015;159:163–9. doi:10.1016/j.clim.2015.03.019.
Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos rep. 2015;13:125–30. doi:10.1007/s11914-015-0257-0.
Al-Asmakh M, Zadjali F. Use of germ-free animal models in microbiota-related research. J Microbiol Biotechnol. 2015;25:1583–8. doi:10.4014/jmb.1501.01039.
•• Sjogren K, et al. The gut microbiota regulates bone mass in mice. J Bone Miner res. 2012;27:1357–67. doi:10.1002/jbmr.1588. This is the first study using germ-free mice to investigate the effect of microbiota on bone remodeling and to suggest a link between microbiota-mediated effects on the immune system and a pro-osteoclastogenic bone marrow microenvironment.
•• Yan J, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S a. 2016;113:E7554–63. doi:10.1073/pnas.1607235113. This study comprehensively evaluates the bone phenotype of both germ-free mice colonized with conventional microbiota and SPF mice treated with antibiotics and demonstrates that microbiota promote both bone formation and resorption with the net effect on bone depending on duration of colonization. These studies further suggest that the effects of microbiota on bone are mediated by induction of systemic IGF-1, possibly by SCFA.
•• Li JY, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126:2049–63. doi:10.1172/JCI86062. This study demonstrates links between sex hormone deficiency, decreased gut permeability, and pro-osteoclastogenic cytokine production. It also provides data suggesting beneficial effects of probiotics on bone loss caused by sex steroid deprivation.
•• Schwarzer M, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–7. doi:10.1126/science.aad8588. This study demonstrated that neonatal growth and systemic IGF-1 are greater in SPF mice compared to germ-free mice. Further, they identified that monocolonization with a specific bacterial strain is sufficient to alter the growth hormone-IGF-1 axis and positively impact bone growth in mice under conditions of undernutrition.
Cox LM, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–21. doi:10.1016/j.cell.2014.05.052.
Rausch P, et al. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. Int J med Microbiol. 2016;306:343–55. doi:10.1016/j.ijmm.2016.03.004.
Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat rev Immunol. 2004;4:478–85. doi:10.1038/nri1373.
Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18:2. doi:10.1186/s12865-016-0187-3.
Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14:449–61. doi:10.1007/s11605-009-1045-x.
Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol rev. 2016;40:117–32. doi:10.1093/femsre/fuv036.
Sommer F, Backhed F. The gut microbiota—masters of host development and physiology. Nat rev Microbiol. 2013;11:227–38. doi:10.1038/nrmicro2974.
Backhed F, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–22. doi:10.1016/j.chom.2012.10.012.
Morgun A, et al. Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut. 2015;64:1732–43. doi:10.1136/gutjnl-2014-308820.
Cho I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6. doi:10.1038/nature11400.
Lundberg R, Toft MF, August B, Hansen AK, Hansen CH. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes. 2016;7:68–74. doi:10.1080/19490976.2015.1127463.
Nobel YR, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. 2015;6:7486. doi:10.1038/ncomms8486.
Guss JD, et al. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner res. 2017; doi:10.1002/jbmr.3114.
Kim D, Yoo SA, Kim WU. Gut microbiota in autoimmunity: potential for clinical applications. Arch Pharm res. 2016;39:1565–76. doi:10.1007/s12272-016-0796-7.
McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228:1793–8. doi:10.1002/jcp.24340.
Collins FL, et al. Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS One. 2016;11:e0153180. doi:10.1371/journal.pone.0153180.
Britton RA, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229:1822–30. doi:10.1002/jcp.24636.
Ohlsson C, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9:e92368. doi:10.1371/journal.pone.0092368.
Parvaneh K, et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed res Int. 2015;2015:897639. doi:10.1155/2015/897639.
Parvaneh K, Jamaluddin R, Karimi G, Erfani R. Effect of probiotics supplementation on bone mineral content and bone mass density. ScientificWorldJournal. 2014;2014:595962. doi:10.1155/2014/595962.
Zhang J, et al. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–82. doi:10.1210/EN.2015-1308.
Storelli G, et al. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–14. doi:10.1016/j.cmet.2011.07.012.
Blanton, L. V. et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016; 351: doi:10.1126/science.aad3311.
Iwami K, Moriyama T. Effects of short chain fatty acid, sodium butyrate, on osteoblastic cells and osteoclastic cells. Int J BioChemiPhysics. 1993;25:1631–5.
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45. doi:10.1016/j.cell.2016.05.041.
Jones D, Glimcher LH, Aliprantis AO. Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J Clin Invest. 2011;121:2534–42. doi:10.1172/JCI46262.
Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–27. doi:10.1016/j.immuni.2010.06.001.
Tan TG, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci U S a. 2016;113:E8141–50. doi:10.1073/pnas.1617460113.
Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi:10.1126/science.1241165.
Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi:10.1038/nature12726.
Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. doi:10.1038/nature12721.
Wesemann DR. Microbes and B cell development. Adv Immunol. 2015;125:155–78. doi:10.1016/bs.ai.2014.09.005.
Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–78. doi:10.1016/j.psyneuen.2012.03.007.
Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos rep. 2005;3:98–102.
Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76. doi:10.1016/j.cell.2015.02.047.
Brommage R, et al. Adult Tph2 knockout mice without brain serotonin have moderately elevated spine trabecular bone but moderately low cortical bone thickness. Bonekey rep. 2015;4:718. doi:10.1038/bonekey.2015.87.
Chabbi-Achengli Y, et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc Natl Acad Sci U S a. 2012;109:2567–72. doi:10.1073/pnas.1117792109.
Cui Y, et al. Lrp5 functions in bone to regulate bone mass. Nat med. 2011;17:684–91. doi:10.1038/nm.2388.
Hernandez CJ, Guss JD, Luna M, Goldring SR. Links between the microbiome and bone. J Bone Miner res. 2016;31:1638–46. doi:10.1002/jbmr.2887.
Clements, S. J. & Carding, S. R. Diet, the intestinal microbiota and immune health in ageing. Crit Rev Food Sci Nutr. 2016; 0: doi:10.1080/10408398.2016.1211086.
LeBlanc JG, et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160–8. doi:10.1016/j.copbio.2012.08.005.
Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi:10.1038/nature08821.
Human Microbiome Project. C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. doi:10.1038/nature11234.
Carlucci C, Petrof EO, Allen-Vercoe E. Fecal microbiota-based therapeutics for recurrent Clostridium Difficile infection, ulcerative colitis and obesity. EBioMedicine. 2016;13:37–45. doi:10.1016/j.ebiom.2016.09.029.
Funding Sources
This work was supported by NIH grants AG046257 from the NIA, AR062590 from the NIAMS, a Faculty Career Development Award from the Brigham and Women’s Hospital, and the Bettina Looram Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Julia Charles and Jing Yan declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Osteoimmunology
Rights and permissions
About this article
Cite this article
Yan, J., Charles, J.F. Gut Microbiome and Bone: to Build, Destroy, or Both?. Curr Osteoporos Rep 15, 376–384 (2017). https://doi.org/10.1007/s11914-017-0382-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-017-0382-z