Abstract
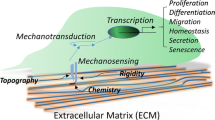
Bone marrow-derived multipotent stem and stromal cells (MSCs) are likely candidates for cell-based therapies for various conditions including skeletal disease. Advancement of these therapies will rely on an ability to identify, isolate, manipulate, and deliver stem cells in a safe and effective manner. Although it is clear that physical signals affect tissue morphogenesis, stem cell differentiation, and healing processes, integration of mechanically induced signaling events remain obscure. Mechanisms underlying sensation and interpretation of mechanical signals by stem cells are the focus of intense study. External mechanical signals have the ability to activate osteogenic signaling pathways in MSCs including Wnt, Ror2, and Runx2. It is also clear that intracellular tensile forces resulting from cell–extracellular matrix interactions play a critical role in MSC regulation. Further work is required to determine the precise role that mechanical forces play in stem cell function.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Recker R, Lappe J, Davies KM, Heaney R: Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res 2004, 19:1628–1633.
Weissman IL, Anderson DJ, Gage F: Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 2001, 17:387–403.
Pan G, Thomson JA: Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res 2007, 17:42–49.
Kuhn NZ, Tuan RS: Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol 2009, 222:268–277.
Shenghui H, Nakada D, Morrison SJ: Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol 2009, 25:377–406.
Siegel G, Schafer R, Dazzi F: The immunosuppressive properties of mesenchymal stem cells. Transplantation 2009, 87(9 Suppl):S45–S49.
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP: Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6:230–247.
Goshima J, Goldberg VM, Caplan AI: The osteogenic potential of culture-expanded rat marrow mesenchymal cells assayed in vivo in calcium phosphate ceramic blocks. Clin Orthop Relat Res 1991, (262):298–311
Conboy IM, Conboy MJ, Wagers AJ, et al.: Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433:760–764.
Song L, Webb NE, Song Y, Tuan RS: Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells 2006, 24:1707–1718.
Dominici M, Paolucci P, Conte P, Horwitz EM: Heterogeneity of multipotent mesenchymal stromal cells: from stromal cells to stem cells and vice versa. Transplantation 2009, 87(9 Suppl):S36–S42.
Chamberlain G, Wright K, Rot A, et al.: Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS One 2008, 3:e2934.
Dominici M, Le Blanc K, Mueller I, et al.: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8:315–317.
Vogel V, Sheetz M: Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 2006, 7:265–275.
Matthews BD, Overby DR, Mannix R, Ingber DE: Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 2006, 119(Pt 3):508–518.
•• Ruiz SA, CS Chen: Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 2008, 26:2921–2927. This study shows that mechanical force gradients can regulate lineage commitment resulting in spatial patterning of stem cell differentiation.
Castillo AB, Jacobs CR: Skeletal mechanobiology. In Mechanobiology Handbook. Edited by Nagatomi J. Boca Raton: CRC Press; 2010 (in press).
Wozniak MA, Chen CS: Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 2009, 10:34–43.
Yamamoto K, Sokabe T, Watabe T, et al.: Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol 2005, 288:H1915–H1924.
Grellier M, Bareille R, Bourget C, Amedee J: Responsiveness of human bone marrow stromal cells to shear stress. J Tissue Eng Regen Med 2009, 3:302–309.
Hanson AD, Marvel SW, Bernacki SH, et al.: Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng 2009, 37:955–965.
Zhang J, Wang JH: Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res 2009 Nov 13 [Epub ahead of print].
Hall BK, Herring SW: Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol 1990, 206:45–56.
Ingber DE: Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol 2006, 50:255–266.
Klein-Nulend J, Veldhuijzen JP, Burger EH: Increased calcification of growth plate cartilage as a result of compressive force in vitro. Arthritis Rheum 1986, 29:1002–1009.
Stokes IA, Aronsson DD, Dimock AN, et al.: Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthop Res 2006, 24:1327–1334.
Warden SJ, Fuchs RK, Castillo AB, et al.: Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res 2007, 22:251–259.
Shimizu N, Yamamoto K, Obi S, et al.: Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor beta. J Appl Physiol 2008, 104:766–772.
Pillarisetti A, Ladjal H, Ferreira A, et al.: Mechanical characterization of mouse embryonic stem cells. Conf Proc IEEE Eng Med Biol Soc 2009, 1:1176–1179.
•• Chowdhury F, Na S, Li D, et al.: Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater 2010, 9:82–88. This study shows that cyclic stress induces cell spreading in nondifferentiated mouse ES cells but not in differentiated ES cells. It demonstrates that cell stiffness regulates cellular mechanosensitivity.
• Haudenschild AK, Hsieh AH, Kapila S, Lotz JC: Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann Biomed Eng 2009, 37:492–502. This study shows that dynamic compressive forces enhance gene expression associated with chondrogenesis, and tensile forces enhance gene expression associated with osteogenesis in human MSCs.
Huang CH, Chen MH, Young TH, et al.: Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem 2009, 108:1263–1273.
Sen B, Xie Z, Case N, et al.: Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 2008, 149:6065–6075.
Wagner DR, Lindsey DP, Li KW, et al.: Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng 2008, 36:813–820.
Doyle AM, Nerem RM, Ahsan T: Human mesenchymal stem cells form multicellular structures in response to applied cyclic strain. Ann Biomed Eng 2009, 37:783–793.
•• Rubin CT, Capilla E, Luu YK, et al.: Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A 2007, 104:17879–17884. This study provides convincing in vivo evidence that mechanical loading inhibits adipogenesis.
McBeath R, Pirone DM, Nelson CM, et al.: Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004, 6:483–495.
• Arnsdorf E, Tummala P, Jacobs CR: Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS ONE 2009, 4:e5388. This study shows that oscillatory fluid flow regulates nuclear translocation of β-catenin and enhances expression of Wnt5a and Ror2 in multipotent cells, both of which are necessary for RhoA activation. RhoA is essential for stress fiber formation in response to mechanical forces.
Arnsdorf E, Tummala P, Kwon RY, Jacobs CR: Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci 2009, 122(Pt 4):546–553.
Sharp LA, Lee YW, Goldstein AS: Effect of low-frequency pulsatile flow on expression of osteoblastic genes by bone marrow stromal cells. Ann Biomed Eng 2009, 37:445–453.
Kearney EM, Prendergast PJ, Campbell VA: Mechanisms of strain-mediated mesenchymal stem cell apoptosis. J Biomech Eng 2008, 130:061004.
Li YJ, Batra NN, You L, et al.: Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res 2004, 22:1283–1289.
Riddle RC, Taylor AF, Genetos DC, Donahue HJ: MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol 2006, 290:C776–C784.
Ghazanfari S, Tafazzoli-Shadpour M, Shokrgozar MA: Effects of cyclic stretch on proliferation of mesenchymal stem cells and their differentiation to smooth muscle cells. Biochem Biophys Res Comm 2009, 388:601–605.
Holtorf HL, Jansen JA, Mikos AG: Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A 2005, 72:326–334.
Datta N, Pham QP, Sharma U, et al.: In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A 2006, 103:2488–2493.
Bjerre L, Bunger CE, Kassem M, Mygind T: Flow perfusion culture of human mesenchymal stem cells on silicate-substituted tricalcium phosphate scaffolds. Biomaterials 2008, 29:2616–2627.
Sandino C, Planell JA, Lacroix D: A finite element study of mechanical stimuli in scaffolds for bone tissue engineering. J Biomech 2008, 41:1005–1014.
Goodship AE, Kenwright J: The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br 1985, 67:650–655.
• Leucht P, Kim JB, Wazen R, et al.: Effect of mechanical stimuli on skeletal regeneration around implants. Bone 2007, 40:919–930. This article shows that mechanical stimulation of a tibial monocortical implant in mice enhances bone healing.
Acknowledgments
We thank Kris Morrow for his assistance with illustrations. This work was supported by a Department of Veterans Affairs Career Development Award (to Dr. Alesha B. Castillo) and a New York State Stem Cell Research Grant N08G-210 (to Dr. Christopher R. Jacobs).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castillo, A.B., Jacobs, C.R. Mesenchymal Stem Cell Mechanobiology. Curr Osteoporos Rep 8, 98–104 (2010). https://doi.org/10.1007/s11914-010-0015-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-010-0015-2