Abstract
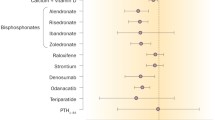
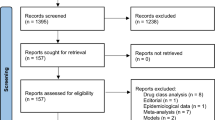
Osteoporosis is a systemic skeletal disorder characterized by low bone mass and microarchitectural deterioration of bone tissue resulting in compromised bone strength and an increased risk of fracture. There are two major fracture types: vertebral and nonvertebral. The latter include fractures involving the upper extremities, lower extremities (including hip), pelvis, and ribs. A recent review comparing the efficacy and safety of drugs approved by the US Food and Drug Administration for treatment of osteoporosis demonstrated that although all of the agents have been shown to reduce the incidence of radiographic vertebral fractures, they do not all reduce the incidence of nonvertebral fractures. This article summarizes the most currently available data relative to nonvertebral and hip fracture risk reduction for the N-containing bisphosphonates alendronate, ibandronate, risedronate, and zoledronic acid, and presents results of an analysis of comparative efficacy of these compounds using the technique of adjusted indirect comparisons.
Similar content being viewed by others
References and Recommended Reading
Consensus Development Conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993, 94:646–650.
Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 2000, 17:1–45.
Chrischilles DA, Butler CD, Davis CS, et al.: A model of lifetime osteoporosis impact. Arch Intern Med 2001, 151:2026–2032.
Johnell O, Kanis JA, Oden A, et al.: Mortality after osteoporosis fractures. Osteoporos Int 2004, 15:38–42.
Seeley DG, Browner WS, Nevitt MC, et al.: Which fractures are associated with low appendicular bone mass in elderly women? The Study of Osteoporotic Fractures Research Group. Ann Intern Med 1991, 115:837–842.
Stone KL, Seeley DG, Lui LY, et al.: BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 2003, 18:1947–1954.
MacLean C, Alexander A, Carter J, et al.: Comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Comparative Effectiveness Review No. 12. (Prepared by Southern California/RAND Evidence-Based Practice Center under contract no. 290-02-0003.) Agency for Healthcare Research and Quality. Available at: http://www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed January 8, 2008.
MacLean C, Newberry S, Maglione M, et al.: Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008, 148:197–213.
Cranney A, Wells G, Willan A, et al.: Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev 2002, 23:508–516.
Cranney A, Tugwell P, Adachi J, et al.: Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 2002, 23:517–523.
Papapoulos SE, Quandt SA, Liberman UA, et al.: Metaanalysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int 2005, 16:468–474.
Stevenson M, Jones ML, De Nigris E, et al.: A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess 2005, 9:1–160.
Nguyen ND, Eisman JA, Nguyen TV: Anti-hip fracture efficacy of bisphosphonates: a Bayesian analysis of clinical trials. J Bone Miner Res 2006, 21:340–349.
Boonen S, Laan RF, Barton IP, Watts NB: Effect of osteoporosis treatments on risk of non-vertebral fractures: review and meta-analysis of intention-to-treat studies. Osteoporos Int 2005, 16:1291–1298.
Liberman UA, Hochberg MC, Geusens P, et al.: Hip and non-spine fracture risk reductions differ among antiresorptive agents: evidence from randomized controlled trials. Int J Clin Pract 2006, 60:1394–1400.
Chesnut CH III, Skag A, Christiansen C, et al.: Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004, 19:1241–1249.
Recker R, Stakkestad JA, Chesnut CH 3rd, et al.: Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone 2004, 34:890–899.
Harris ST, Blumentals WA, Miller PD: Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Curr Med Res Opin 2008, 24:237–245.
Black DM, Delmas PD, Eastell R, et al.: Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007, 356:1809–1822.
Lyles KW, Colon-Emeric CS, Magaziner JS, et al.: Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007, 357:1799–1809.
Wells G, Cranney A, Peterson J, et al.: Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2008, 23:CD001155.
Wells G, Cranney A, Peterson J, et al.: Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2008, 23:CD004523.
Bucher HC, Guyatt GH, Griffith LE, Walter SD: The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997, 50:683–691.
Schnitzer T, Bone HG, Crepaldi G, et al.: Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano) 2000, 12:1–12.
Rizzoli R, Greenspan SL, Bone HG, et al.: Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res 2002, 17:1988–1996.
Brown JP, Kendler DL, McClung MR, et al.: The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 2002, 71:103–111.
Harris ST, Watts NB, Li Z, et al.: Two-year efficacy and tolerability of risedronate once a week for the treatment of women with postmenopausal osteoporosis. Curr Med Res Opin 2004, 20:757–764.
Delmas PD, McClung MR, Zanchetta JR, et al.: Efficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosis. Bone 2008, 42:36–42.
Delmas PD, Benhamou CL, Man Z, et al.: Monthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety results. Osteoporos Int 2008, 19:1039–1045.
Miller PD, McClung MR, Macovei L, et al.: Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from MOBILE study. J Bone Miner Res 2005, 20:1315–1322.
Reginster JY, Adami S, Lakatos P, et al.: Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2-year results from the MOBILE study. Ann Rheum Dis 2006, 65:654–661.
Delmas PD, Adami S, Strugala C, et al.: Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum 2006, 54:1838–1846.
Eisman JA, Civitelli R, Adami S, et al.: Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 2008, 35:488–497.
Papapoulos SE, Schimmer RC: Changes in bone remodeling and antifracture efficacy of intermittent bisphosphonate therapy: implications from clinical studies with ibandronate. Ann Rheum Dis 2007, 66:853–858.
McAlister FA, Laupacis A, Wells GA, Sackett DL, for the Evidence-Based Medicine Working Group: Users’ Guides to the Medical Literature: XIX. Applying clinical trial results B. Guidelines for determining whether a drug is exerting (more than) a class effect. JAMA 1999, 282:1371–1377.
Hosking D, Adami S, Felsenberg D, et al.: Comparison of change in bone resorption and bone mineral density with once-weekly alendronate and daily risedronate: a randomized, placebo-controlled study. Curr Med Res Opin 2003, 19:383–394.
Rosen CJ, Hochberg MC, Bonnick SL, et al.: Treatment with once-weekly alendronate 70 mg compared with onceweekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 2005, 20:141–151.
Bonnick S, Saag KG, Kiel DP, et al.: Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 2006, 91:2631–2637.
Miller PD, Epstein S, Sedarati F, Reginster JY: Oncemonthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION study. Curr Med Res Opin 2008, 24:207–213.
Hochberg MC, Greenspan S, Wasnich RD, et al.: Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 2002, 87:1586–1592.
Hochberg MC: The comparative efficacy of N-containing bisphosphonates as measured by fracture risk reduction [abstract]. Abstract submitted for 2008 Annual Meeting of the American Society for Bone and Mineral Research.
Miller PD: Non-vertebral fracture risk reduction with oral bisphosphonates: challenges with interpreting clinical trial data. Curr Med Res Opin 2008, 24:107–119.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hochberg, M.C. Nonvertebral fracture risk reduction with nitrogen-containing bisphosphonates. Curr Osteoporos Rep 6, 89–94 (2008). https://doi.org/10.1007/s11914-008-0016-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-008-0016-6