Abstract
Teriparatide, recombinant human parathyroid hormone (1-34) (rhPTH [1-34]), is approved for the treatment of osteoporosis in men and postmenopausal women at high risk for fracture. The best candidates are those who have already had vertebral compression fractures (symptomatic or asymptomatic) or other osteoporosis-related fractures, or those who have very low bone mineral density, in the T score range of -3.5 or below. Teriparatide is the first anabolic drug approved by the US Food and Drug Administration for osteoporosis. It not only dramatically improves bone mass, but also restores bone microarchitecture and increases bone diameter. All of these mechanisms contribute to increasing bone strength and reducing the risk for osteoporosis-related fractures. Although PTH has been used in combination with other agents such as estrogens, calcitonin, and bisphosphonates, the relative benefit of the combined approach versus teriparatide alone for fracture risk reduction has not been shown. In fact, some data suggest that initiating PTH and alendronate together in previously untreated patients or pretreating patients for a short time with alendronate before initiating PTH may somewhat reduce the anabolic response to PTH. There are many unanswered questions regarding PTH, such as the optimal duration of treatment, the optimal sequence of medications for severe osteoporosis, the mechanism of resistance to effect after 18 to 24 months, the effect of subsequent rechallenge with PTH and, most importantly, surrogates to measure PTH effect.
Similar content being viewed by others
References and Recommended Reading
Bauer W, Aub JC, Albright F: Studies of calcium phosphorus metabolisms: study of bone trabeculae as ready available reserve supply of calcium. J Exp Med 1929, 49:145–162.
Selye H: On the stimulation of new bone-formation with parathyroid extract and irradiated ergosterol. Endocrinology 1932, 16:547–558.
Tam CS, Heersche JNM, Murray TM, et al.:Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 1982, 110:506–512.
Podbesek R, Edouard C, Peunier PJ, et al.:Effects of two treatment regimes with synthetic human parathyroid hormone fragment on bone formation and the tissue balance of trabecular bone in greyhounds. Endocrinology 1983, 112:1000–1006.
Dobnig H, Turner RT: The effects of programmed administration of human parathyroid hormone fragment (1-34) on bone histomorphometry and serum chemistry in rats. Endocrinology 1997, 138:4607–4612.
Cosman F, Shen V, Xie F, et al.:Estrogen protection against bone resorbing effects of parathyroid hormone infusion. Ann Intern Med 1993, 118:337–343.
Lindsay R, Nieves J, Henneman E, et al.:Subcutaneous administration of the amino terminal fragment of human parathyroid hormone (1-34hPTH): kinetics and biochemical response in estrogenized osteoporotic patients. J Clin Endocrinol Metab 1993, 77:1535–1539.
Ma YL, Cain RL, Halladay DL, et al.:Catabolic effects of continuous human PTH (1-38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoportegein and gene-associated bone formation. Endocrinology 2001, 142:4047–4054.
Hock JM: Anabolic actions of PTH in the skeletons of animals. Musculoskel Neuron Interact 2001, 2:33–47.
Jilka RL,Weinstein RS,Bellido T, et al.:Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 1999, 104:439–446.
Dempster DW,Cosman F,Parisien M, et al.:Anabolic actions of parathyroid hormone on bone. Update 1995. Endocr Rev 1995, 4:247–250.
Cosman F,Lindsay R: Is parathyroid hormone a therapeutic option for osteoporosis? A review of the clinical evidence. Calcif Tissue Int 1998, 62:475–480.
Cosman F,Nieves J,Woelfert L, et al.:Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res 2001, 16:925–931. Fifty-two women with postmenopausal osteoporosis, previously treated with HT were randomized to receive daily PTH (1-34) with HT or continue HT alone. Spine BMD increased approximately 14% and vertebral deformity incidence decreased dramatically over 3 years in the PTH plus HT group.
Lindsay R,Nieves J,Formica C, et al.:Randomized controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997, 350:550–555.
Roe EB,Sanchez SD,del Puerto GA, et al.:Parathyroid hormone 1–34 (hPTH 1–34) and estrogen produce dramatic bone density increases in postmenopausal osteoporosis — results from a placebo-controlled randomized trial. J Bone Miner Res 1999, 12(suppl 1):S137.
Ste-Marie LG, Scheele WH, et al.:Effect of LY333334 [recombinant human parathyroid hormone (1-34), rhPTH(1-34)] on bone density when given to postmenopausal women receiving hormone replacement therapy (HRT) [abstract]. J Clin Endocrinol Metab 2001, (suppl):39509.
Kurland ES,Cosman F,McMahon DJ, et al.:Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 2000, 85:3069–3076.
Hodsman AB, Lindsay R: Effects of 1–84 PTH over 1 year. J Clin Endocrinol Metab 2003, In press. In this study of PTH (1-84) in 217 postmenopausal women, there was a dose dependent increase in spine BMD and spine area over 1 year with smaller and less consistent changes in the hip. No significant change in total body bone was seen. The study was not powered to assess fracture outcomes.
Neer RM,Arnaud CD,Zanchetta JR, et al.:Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001, 344:1434–1441. Daily teriparatide was given in one of two doses (20 μcg or 40 μcg) versus placebo in this randomized clinical trial in 1637 postmenopausal women with osteoporosis, the largest PTH trial to date. In PTH treated women, BMD increased at all sites (except the radius) and fracture incidence was reduced throughout the skeleton, approximately 65% in the spine and 40% in the nonvertebral skeleton (20 μcg dose).
Vahle JL,Sato M,Long GG, et al.:Skeletal changes in rats given daily subcutaneous injections of rhPTH(1-34) for 2 years and relevance to human safety. Toxicol Pathol 2002, 30:312–321.
Body J-J,Gaich GA,Scheele WH, et al.:A randomized doubleblind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002, 87:4528–4535.
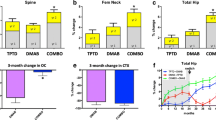
Black DM,Rosen CM,Greenspan S, et al.:The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003, 349:1207–1215. Two hundred thirty-eight previously untreated women at high risk for fracture were randomly assigned to receive daily PTH (1-84), alendronate or combined PTH plus alendronate. For some of the outcomes measured, PTH alone was superior to PTH plus alendronate. There was a very low incidence of fracture in all groups and no differences among groups.
Neer RM,Hayes A,Rao A, et al.:Effects of parathyroid hormone, alendronate, or both on bone density in osteoporotic postmenopausal women. J Bone Miner Res 2002, 17(suppl 1):S135.
Cosman F,Nieves J,Woelfert L, et al.:Alendronate does not block the anabolic effect of PTH in postmenopausal osteoporotic women. J Bone Miner Res 1998, 13:1051–1055.
Cosman F,Nieves JW,Luckey M, et al.:Daily versus cyclic PTH combined with alendronate versus alendronate alone for treatment of osteoporosis. J Bone Miner Res 2003, 18(suppl 2):S33.
Ettinger B,San Martin JA, Crans G, et al.:Early response of bone turnover markers and bone density to teriparatide [recombinant human parathyroid hormone (1-34)] in postmenopausal women previously treated with an antiresorptive drug. J Bone Miner Res 2002, 17(suppl 1):S157.
Ettinger B,San Martin J,Crans GG, et al.:Response of markers of bone turnover and bone density to teriparatide in postmenopausal women previously treated with an antiresorptive drug. J Bone Miner Res 2003, 18(suppl 2):1055.
Orwoll ES,Scheele WH,Paul S, et al.:The effects of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 2003, 18:9–17. Four hundred thirty-seven men at risk for fracture were randomly assigned to teriparatide 20 μcg, teriparatide 40 μcg, or placebo. BMD increased in the spine, hip, and total body with higher doses having greater gains. Vertebral deformity incidence was reduced in PTH treated men.
Orwoll ES,Scheele WH,Paul S, et al.:Recombinant human parathyroid hormone (1-34) therapy reduces the incidence of moderate/severe vertebral fractures in men with lost bone density. J Bone Miner Res 2001, 16(suppl 1):S162.
Finkelstein JS,Hayes A,Hunzelman JL, et al.:The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 2003, 349:1216–1226.
Lane NE,Sanchez S,Modin GW, et al.:Bone mass continues to increase at the hip after parathyroid hormone treatment is discontinued in glucocorticoid-induced osteoporosis: results of a randomized controlled clinical trial. J Bone Miner Res 2000, 15:944–951.
Finkelstein JS,Klibanski A,Arnold AL, et al.:Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1-34): a randomized, controlled trial. JAMA 1998, 280:1067–1073.
Lindsay R,Zhou H,Cosman F: Short term response to parathyroid hormone (1-34hPTH) in human iliac crest bone using a unique quadruple (double double) tetracycline labeling regimen and single biopsy [abstract 1208]. Proceedings of the ASBMR 25th Annual Meeting. Minneapolis: 2003:18(suppl 2):S54.
Dempster DW,Cosman F,Kurland ES, et al.:Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 2001, 16:1846–1853.
Burr DB,Hirano T,Turner CH, et al.:Intermittently administered human parathyroid hormone (1-34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res 2001, 16:157–165.
Lindsay R,Cosman F,Nieves J, et al.:Does treatment with PTH alter vertebral size? Osteoporos Int 2000, 11(suppl 2):556.
Rehman Q,Lang TF,Arnaud CD, et al.:Daily treatment with parathyroid hormone is associated with an increase in vertebral cross-sectional area in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int 2003, 14:77–81.
Zanchetta JR,Bogado C,Ferretti JL, et al.:Effects of teriparatide [recombinant parathyroid hormone (1-34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res 2003, 18:539–543.
Hyldstrup L,Jorgensen JT, et al.:Assessment of effects of LY333334 [recombinant human parathyroid hormone (1-34)] on cortical bone using digital x-ray radiogrammetry. Bone 2001, 28(suppl 5):S97-S98.
Rittmaster RS,Bolognese M,Ettinger MP, et al.:Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 2000, 85:2129–2134.
Kurland ES,Heller SL,Cosman F, et al.:The post-PTH experience in men with idiopathic osteoporosis: bisphosphonates vs. non-pharmacological therapy [abstract F363]. J Bone Miner Res 2001, 16(supp 1):S219.
Lindsay R, Ste-Marie LG, Scheele WH, et al.: Bone mineral density changes during an 18 month observational period following discontinuation of recombinant human parathyroid hormone (1-34) treatment of postmenopausal women receiving hormone replacement therapy. JAMA 2004, In press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cosman, F., Lindsay, R. Therapeutic potential of parathyroid hormone. Curr Osteoporos Rep 2, 5–11 (2004). https://doi.org/10.1007/s11914-004-0008-0
Issue Date:
DOI: https://doi.org/10.1007/s11914-004-0008-0