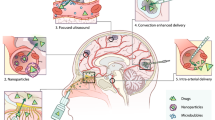
Abstract
Purpose of Review
To review relevant advances in the past half-decade in the treatment of primary brain tumors via modification of blood–brain barrier (BBB) permeability.
Recent Findings
BBB disruption is becoming increasingly common in the treatment of primary brain tumors. Use of mannitol in BBB disruption for targeted delivery of chemotherapeutics via superselective intra-arterial cerebral infusion (SIACI) is the most utilized strategy to modify the BBB. Mannitol is used in conjunction with chemotherapeutics, oligonucleotides, and other active agents. Convection-enhanced delivery has become an attractive option for therapeutic delivery while bypassing the BBB. Other technologic innovations include laser interstitial thermal therapy (LITT) and focused ultrasound (FUS) which have emerged as prime modalities to directly target tumors and cause significant local BBB disruption.
Summary
In the past 5 years, interest has significantly increased in studying modalities to disrupt the BBB in primary brain tumors to enhance treatment responses and improve clinical outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7: a020412.
Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14.
Dowden H, Munro J. Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov. 2019;18:495–6.
van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12.
Steeg PS. The blood-tumour barrier in cancer biology and therapy. Nat Rev Clin Oncol. 2021;18:696–714.
Whelan R, Hargaden GC, Knox AJS. Modulating the blood-brain barrier: a comprehensive review. Pharmaceutics. 2021;13(11):1980. https://doi.org/10.3390/pharmaceutics13111980.
Wang D, Wang C, Wang L, Chen Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019;26:551–65.
Puratchikody A, Prabu SL, Umamaheswari A. Computer applications in drug discovery and development. IGI Global; 2018.
Löscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98.
Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215.
Rapoport SI, Hori M, Klatzo I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am J Physiol. 1972;223:323–31.
Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26–41.
Noorani B, Bhalerao A, Raut S, Nozohouri E, Bickel U, Cucullo L. A Quasi-physiological microfluidic blood-brain barrier model for brain permeability studies. Pharmaceutics. 2021;13(9):1474. https://doi.org/10.3390/pharmaceutics13091474.
Linville RM, DeStefano JG, Sklar MB, Chu C, Walczak P, Searson PC. Modeling hyperosmotic blood-brain barrier opening within human tissue-engineered in vitro brain microvessels. J Cereb Blood Flow Metab. 2020;40:1517–32.
Linville RM, DeStefano JG, Sklar MB, Xu Z, Farrell AM, Bogorad MI, et al. Human iPSC-derived blood-brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials. 2019;190–191:24–37.
Wang M, Etu J, Joshi S. Enhanced disruption of the blood brain barrier by intracarotid mannitol injection during transient cerebral hypoperfusion in rabbits. J Neurosurg Anesthesiol. 2007;19:249–56.
Kessler RM, Goble JC, Bird JH, Girton ME, Doppman JL, Rapoport SI, et al. Measurement of blood-brain barrier permeability with positron emission tomography and [68Ga]EDTA. J Cereb Blood Flow Metab. 1984;4:323–8.
Fricker G, Ott M, Mahringer A. The bloo-brain barrier (BBB). Springer; 2014.
• Chu C, Jablonska A, Gao Y, Lan X, Lesniak WG, Liang Y, et al. Hyperosmolar blood-brain barrier opening using intra-arterial injection of hyperosmotic mannitol in mice under real-time MRI guidance. Nat Protoc. 2022;17:76–94. Details extensively the effects that hyperosmolar injections via modern MRI guidance has on BBB opening in an animal model.
• Burks SR, Kersch CN, Witko JA, Pagel MA, Sundby M, Muldoon LL, et al. Blood-brain barrier opening by intracarotid artery hyperosmolar mannitol induces sterile inflammatory and innate immune responses. Proc Natl Acad Sci U S A. 2021;118. https://doi.org/10.1073/pnas.2021915118. Shows that intraarterial mannitol is able to induce immune response.
Choi C, Kim HM, Shon J, Park J, Kim H-T, Oh S-H, et al. Additional increased effects of mannitol-temozolomide combined treatment on blood-brain barrier permeability. Biochem Biophys Res Commun. 2018;497:769–75.
D’Amico RS, Khatri D, Reichman N, Patel NV, Wong T, Fralin SR, et al. Correction to: super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood-brain barrier disruption: where are we now, and where we are going. J Neurooncol. 2020;147:279.
• Kulason KO, Schneider JR, Chakraborty S, Filippi CG, Pramanik B, Wong T, et al. Superselective intraarterial cerebral infusion of cetuximab with blood brain barrier disruption combined with Stupp Protocol for newly diagnosed glioblastoma. J Exp Ther Oncol. 2018;12:223–9. Gave data that injection of cetuximab following mannitol injection in combination of Stupp protocol could be a reasonable treatment method for newly diagnosed EGFR amplified glioblastoma.
Boockvar JA, Tsiouris AJ, Hofstetter CP, Kovanlikaya I, Fralin S, Kesavabhotla K, et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J Neurosurg. 2011;114:624–32.
Jeon JY, Kovanlikaya I, Boockvar JA, Mao X, Shin B, Burkhardt JK, et al. Metabolic response of glioblastoma to superselective intra-arterial cerebral infusion of bevacizumab: a proton MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2012;33:2095–102.
Galla N, Chiang G, Chakraborty S, Singh R, John Tsiouris A, Boockvar J, et al. Apparent diffusion coefficient changes predict survival after intra-arterial bevacizumab treatment in recurrent glioblastoma. Neuroradiology. 2017;59:499–505.
Riina HA, Fraser JF, Fralin S, Knopman J, Scheff RJ, Boockvar JA. Superselective intraarterial cerebral infusion of bevacizumab: a revival of interventional neuro-oncology for malignant glioma. J Exp Ther Oncol. 2009;8:145–50.
• McCrea HJ, Ivanidze J, O’Connor A, Hersh EH, Boockvar JA, Gobin YP, et al. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: results of a phase I trial. J Neurosurg Pediatr. 2021;28:371–9. Showed that intraarterial mannitol and concomitant immunotherapy is safe in pediatric patients.
Iorio-Morin C, Gahide G, Morin C, Vanderweyen D, Roy M-A, St-Pierre I, et al. Management of primary central nervous system lymphoma using intra-arterial chemotherapy with osmotic blood-brain barrier disruption: retrospective analysis of the sherbrooke cohort. Front Oncol. 2020;10: 543648.
Home - ClinicalTrials.gov. [cited 2023 May 10]. Available from: https://clinicaltrials.gov/. Accessed 22 Jan 2024.
Sedeyn JC, Wu H, Hobbs RD, Levin EC, Nagele RG, Venkataraman V. Histamine induces Alzheimer’s disease-like blood brain barrier breach and local cellular responses in mouse brain organotypic cultures. Biomed Res Int. 2015;2015: 937148.
Dahlén SE, Björk J, Hedqvist P, Arfors KE, Hammarström S, Lindgren JA, et al. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci U S A. 1981;78:3887–91.
Wang M-L, Huang X-J, Fang S-H, Yuan Y-M, Zhang W-P, Lu Y-B, et al. Leukotriene D4 induces brain edema and enhances CysLT2 receptor-mediated aquaporin 4 expression. Biochem Biophys Res Commun. 2006;350:399–404.
Lenz QF, Arroyo DS, Temp FR, Poersch AB, Masson CJ, Jesse AC, et al. Cysteinyl leukotriene receptor (CysLT) antagonists decrease pentylenetetrazol-induced seizures and blood-brain barrier dysfunction. Neuroscience. 2014;277:859–71.
Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–47.
Wang Z, Cai X-J, Qin J, Xie F-J, Han N, Lu H-Y. The role of histamine in opening blood-tumor barrier. Oncotarget. 2016;7:31299–310.
Towner RA, Saunders D, Lerner M, Silasi Mansat R, Yuan T, Barber D, et al. Temporary opening of the blood-brain barrier with the nitrone compound OKN-007. Am J Nucl Med Mol Imaging. 2021;11:363–73.
Towner RA, Hocker J, Smith N, Saunders D, Battiste J, Hanas J. OKN-007 alters protein expression profiles in high-grade gliomas: mass spectral analysis of blood sera. Brain Sci. 2022;12(1):100. https://doi.org/10.3390/brainsci12010100.
Sabir F, Ismail R, Csoka I. Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: status quo and outlook. Drug Discov Today. 2020;25:185–94.
Dutta L, Mukherjee B, Chakraborty T, Das MK, Mondal L, Bhattacharya S, et al. Lipid-based nanocarrier efficiently delivers highly water soluble drug across the blood-brain barrier into brain. Drug Deliv. 2018;25:504–16.
Wang W, Marín-Ramos NI, He H, Zeng S, Cho H-Y, Swenson SD, et al. NEO100 enables brain delivery of blood-brain barrier impermeable therapeutics. Neuro Oncol. 2021;23:63–75.
da Fonseca CO, Simão M, Lins IR, Caetano RO, Futuro D, Quirico-Santos T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J Cancer Res Clin Oncol. 2011;137:287–93.
Hashizume R, Ozawa T, Gryaznov SM, Bollen AW, Lamborn KR, Frey WH 2nd, et al. New therapeutic approach for brain tumors: intranasal delivery of telomerase inhibitor GRN163. Neuro Oncol. 2008;10:112–20.
Breitkreuz-Korff O, Tscheik C, Del Vecchio G, Dithmer S, Walther W, Orthmann A, et al. M01 as a novel drug enhancer for specifically targeting the blood-brain barrier. J Control Release. 2021;338:137–48.
Kariolis MS, Wells RC, Getz JA, Kwan W, Mahon CS, Tong R, et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med. 2020;12. https://doi.org/10.1126/scitranslmed.aay1359.
Stocki P, Szary J, Rasmussen CLM, Demydchuk M, Northall L, Logan DB, et al. Blood-brain barrier transport using a high affinity, brain-selective VNAR antibody targeting transferrin receptor 1. FASEB J. 2021;35: e21172.
Galstyan A, Markman JL, Shatalova ES, Chiechi A, Korman AJ, Patil R, et al. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun. 2019;10:3850.
Ye Z, Gastfriend BD, Umlauf BJ, Lynn DM, Shusta EV. Antibody-targeted liposomes for enhanced targeting of the blood-brain barrier. Pharm Res. 2022;39:1523–34.
Godinho BMDC, Henninger N, Bouley J, Alterman JF, Haraszti RA, Gilbert JW, et al. Transvascular delivery of hydrophobically modified sirnas: gene silencing in the rat brain upon disruption of the blood-brain barrier. Mol Ther. 2018;26:2580–91.
Wang Y, Pang J, Wang Q, Yan L, Wang L, Xing Z, et al. Delivering antisense oligonucleotides across the blood-brain barrier by tumor cell-derived small apoptotic bodies. Adv Sci. 2021;8:2004929.
Zeniya S, Kuwahara H, Daizo K, Watari A, Kondoh M, Yoshida-Tanaka K, et al. Angubindin-1 opens the blood-brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J Control Release. 2018;283:126–34.
Kim D-G, Jang M, Choi S-H, Kim H-J, Jhun H, Kim H-C, et al. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery. Int J Biol Macromol. 2018;114:1325–37.
Smith-Cohn MA, Burley NB, Grossman SA. Transient opening of the blood-brain barrier by vasoactive peptides to increase CNS drug delivery: reality versus wishful thinking? Curr Neuropharmacol. 2022;20:1383–99.
Bynoe MS, Viret C, Yan A, Kim D-G. Adenosine receptor signaling: a key to opening the blood-brain door. Fluids Barriers CNS. 2015;12:20.
Carman AJ, Mills JH, Krenz A, Kim D-G, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci. Society for Neuroscience. 2011;31:13272–80.
Kim D-G, Bynoe MS. A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J Clin Invest. American Society for Clinical Investigation; 2016;126:1717–33.
Vézina A, Manglani M, Morris D, Foster B, McCord M, Song H, et al. Adenosine A2A receptor activation enhances blood-tumor barrier permeability in a rodent glioma model. Mol Cancer Res. 2021;19:2081–95.
Jackson S, Weingart J, Nduom EK, Harfi TT, George RT, McAreavey D, et al. The effect of an adenosine A agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS. 2018;15:2.
Jackson S, George RT, Lodge MA, Piotrowski A, Wahl RL, Gujar SK, et al. The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J Neurooncol. 2017;132:513–9.
Jackson S, Anders NM, Mangraviti A, Wanjiku TM, Sankey EW, Liu A, et al. The effect of regadenoson-induced transient disruption of the blood-brain barrier on temozolomide delivery to normal rat brain. J Neurooncol. 2016;126:433–9.
Frøslev P, Franzyk H, Ozgür B, Brodin B, Kristensen M. Highly cationic cell-penetrating peptides affect the barrier integrity and facilitates mannitol permeation in a human stem cell-based blood-brain barrier model. Eur J Pharm Sci. 2022;168: 106054.
Yuan BO, Zhao Y, Dong S, Sun Y, Hao F, Xie J, et al. Cell-penetrating peptide-coated liposomes for drug delivery across the blood-brain barrier. Anticancer Res. 2019;39:237–43.
Neuhaus W, Piontek A, Protze J, Eichner M, Mahringer A, Subileau E-A, et al. Reversible opening of the blood-brain barrier by claudin-5-binding variants of Clostridium perfringens enterotoxin’s claudin-binding domain. Biomaterials. 2018;161:129–43.
Norouzi M, Yathindranath V, Thliveris JA, Miller DW. Salinomycin-loaded iron oxide nanoparticles for glioblastoma therapy. Nanomaterials (Basel). 2020;10(3):477. https://doi.org/10.3390/nano10030477.
Shoffstall AJ, Paiz JE, Miller DM, Rial GM, Willis MT, Menendez DM, et al. Potential for thermal damage to the blood-brain barrier during craniotomy: implications for intracortical recording microelectrodes. J Neural Eng. 2018;15: 034001.
Nian K, Harding IC, Herman IM, Ebong EE. Blood-brain barrier damage in ischemic stroke and its regulation by endothelial mechanotransduction. Front Physiol. 2020;11: 605398.
Belykh E, Shaffer KV, Lin C, Byvaltsev VA, Preul MC, Chen L. Blood-brain barrier, blood-brain tumor barrier, and fluorescence-guided neurosurgical oncology: delivering optical labels to brain tumors. Front Oncol. 2020;10:739.
Kang JH, Desjardins A. Convection-enhanced delivery for high-grade glioma. Neurooncol Pract. 2022;9:24–34.
Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23:89–104.
Mungur R, Zheng J, Wang B, Chen X, Zhan R, Tong Y. Low-intensity focused ultrasound technique in glioblastoma multiforme treatment. Front Oncol. 2022;12: 903059.
•• Chen K-T, Chai W-Y, Lin Y-J, Lin C-J, Chen P-Y, Tsai H-C, et al. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci Adv. 2021;7. https://doi.org/10.1126/sciadv.abd0772. Demonstrated duration of BBB opening after FUS.
Anastasiadis P, Gandhi D, Guo Y, Ahmed A-K, Bentzen SM, Arvanitis C, et al. Localized blood-brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions-controlled focused ultrasound. Proc Natl Acad Sci U S A. 2021;118. https://doi.org/10.1073/pnas.2103280118.
Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. 2019;9:321.
• Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. One-year outcome of multiple blood-brain barrier disruptions with temozolomide for the treatment of glioblastoma. Front Oncol. 2020;10:1663. Provides long term follow up data on patient that have had multiple FUS treatments.
•• Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J Neurosurg. 2020;1–9. Demonstrates that repeated BBB disruption by MRgFUS is safe and feasible in patients with GBM undergoing concomitant standard adjuvant temozolomide chemotherapy.
Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res. 2019;25:3793–801.
Hart E ’t, Odé Z, Derieppe MPP, Groenink L, Heymans MW, Otten R, et al. Blood-brain barrier permeability following conventional photon radiotherapy - a systematic review and meta-analysis of clinical and preclinical studies. Clin Transl Radiat Oncol. 2022;35:44–55.
• Shin DH, Melnick KF, Tran DD, Ghiaseddin AP. In situ vaccination with laser interstitial thermal therapy augments immunotherapy in malignant gliomas. J Neurooncol. 2021;151:85–92. Shows that LITT may be work with immunotherapies by promoting in situ vaccination.
Patel B, Yang PH, Kim AH. The effect of thermal therapy on the blood-brain barrier and blood-tumor barrier. Int J Hyperthermia. 2020;37:35–43.
Morris S-A, Rollo M, Rollo P, Johnson J, Grant GA, Friedman E, et al. Prolonged blood-brain barrier disruption following laser interstitial ablation in epilepsy: a case series with a case report of postablation optic neuritis. World Neurosurg. 2017;104:467–75.
• Butt OH, Zhou AY, Huang J, Leidig WA, Silberstein AE, Chheda MG, et al. A phase II study of laser interstitial thermal therapy combined with doxorubicin in patients with recurrent glioblastoma. Neurooncol Adv. 2021;3:vdab164. Clinical trial data on combination of LITT with doxorubicin showing some improvements in outcomes.
•• Hormigo A, Chiu D, Hahn M, Qi J, Lee B, Mandeli J, et al. Ctim-09. Phase I study of pd-l1 inhibition with avelumab and laser interstitial thermal therapy in patients with recurrent glioblastoma. Neuro Oncol. Oxford University Press (OUP); 2021;23:vi51–vi51. Avelumab and LITT resulted in significant increase in survival.
Campian J, Butt O, Ghinaseddin A, Rahman M, Chheda M, Johanns T, et al. Ctim-26. Phase I/II study of the combination of pembrolizumab (Mk-3475) and laser interstitial thermal therapy (litt) in recurrent glioblastoma. Neuro Oncol. Oxford University Press (OUP); 2021;23:vi56–vi56.
Leuthardt EC, Duan C, Kim MJ, Campian JL, Kim AH, Miller-Thomas MM, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11: e0148613.
Maraka S, Asmaro K, Walbert T, Lee I. Cerebral edema induced by laser interstitial thermal therapy and radiotherapy in close succession in patients with brain tumor. Lasers Surg Med. 2018;50:917–23.
Salem U, Kumar VA, Madewell JE, Schomer DF, de Almeida Bastos DC, Zinn PO, et al. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging. 2019;19:65.
Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: single-center experience with the Visualase thermal therapy system. J Neurosurg. 2016;125:853–60.
Ali SC, Basil GW, Diaz RJ, Komotar RJ. The safety of bevacizumab administered shortly after laser interstitial thermal therapy in glioblastoma: a case series. World Neurosurg. 2018;117:e588–94.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Virtanen, P.S., Ortiz, K.J., Patel, A. et al. Blood–Brain Barrier Disruption for the Treatment of Primary Brain Tumors: Advances in the Past Half-Decade. Curr Oncol Rep 26, 236–249 (2024). https://doi.org/10.1007/s11912-024-01497-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-024-01497-7