Abstract
Purpose of Review
While vascular endothelial growth factor receptor inhibitors (VEGFRis) have dramatically improved cancer survival, these drugs cause hypertension in a majority of patients. This side effect is often dose limiting and increases cardiovascular mortality in cancer survivors. This review summarizes recent advances in our understanding of the molecular mechanisms and clinical findings that impact management of VEGFRi-induced hypertension.
Recent Findings
Recent studies define new connections between endothelial dysfunction and VEGFRi-induced hypertension, including the balance between nitric oxide, oxidative stress, endothelin signaling, and prostaglandins and the potential role of microparticles, vascular smooth muscle cells, vascular stiffness, and microvessel rarefaction. Data implicating genetic polymorphisms that might identify patients at risk for VEGFRi-induced hypertension and the growing body of literature associating VEGFRi-induced hypertension with antitumor efficacy are reviewed.
Summary
These recent advances have implications for the future of cardio-oncology clinics and the management of VEGFRi-induced hypertension.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. Heart. 2018;104:1995–2002.
Neves KB, Montezano AC, Lang NN, Touyz RM. Vascular toxicity associated with anti-angiogenic drugs. Clin Sci (Lond). 2020;134:2503–20.
Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precision Onc. 2018;2:13.
Yang B, Papoian T. Preclinical approaches to assess potential kinase inhibitor-induced cardiac toxicity: past, present and future. J Appl Toxicol. 2018;38:790–800.
Akam-Venkata J, Franco VI, Lipshultz SE. Late cardiotoxicity: issues for childhood cancer survivors. Curr Treat Options Cardio Med. 2016;18:47.
Gopal S, Miller KB, Jaffe IZ. Molecular mechanisms for vascular complications of targeted cancer therapies. Clin Sci. 2016;130:1763–79.
Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–67.
Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47.
• Abdel-Qadir H, Ethier J-L, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treatment Reviews. 2017;53:120–7 (Large meta-analysis quantifying the cardiovascular toxicity of VEGFRi treatment including increased risk of hypertension, cardiac ischemia, cardiac dysfunction, and arterial thromboembolism).
Shah NS, Lloyd-Jones DM, O’Flaherty M, Capewell S, Kershaw K, Carnethon M, Khan SS. Trends in Cardiometabolic Mortality in the United States, 1999–2017. JAMA. 2019;322:780.
Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:1–9.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology challenges and perspectives. Circ Res. 2019;124:1094–112.
Jammal N, Pan E, Hurwitz M, Abramovitz RB. Outcomes of combination therapy with tyrosine kinase inhibitors and immune checkpoint inhibitors in metastatic renal cell carcinoma – a retrospective study. J Oncol Pharm Pract. 2020;26:556–63.
Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34:355–64.
Haller H. Endothelial Function. Drugs. 1997;53:1–10.
Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–25.
Ribatti D, Annese T, Ruggieri S, Tamma R, Crivellato E. Limitations of anti-angiogenic treatment of tumors. Translational Oncology. 2019;12:981–6.
Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–62.
Wirth LJ, Tahara M, Robinson B, et al. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer. 2018;124:2365–72.
Iacovelli RN, Sternberg C, Porta C, Verzoni E, de Braud F, Escudier B, Procopio G. Inhibition of the VEGF/VEGFR pathway improves survival in advanced kidney cancer: a systematic review and meta-analysis. Current Drug Targets. 2015;16:164–70.
Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med. 2012;2:a006577.
Jim B, Karumanchi SA. Preeclampsia: pathogenesis, prevention, and long-term complications. Semin Nephrol. 2017;37:386–97.
London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856–65.
Berger EP, Johannes CM, Jergens AE, Allenspach K, Powers BE, Du Y, Mochel JP, Fox LE, Musser ML. Retrospective evaluation of toceranib phosphate (Palladia®) use in the treatment of gastrointestinal stromal tumors of dogs. J Vet Intern Med. 2018;32:2045–53.
Lew FH, McQuown B, Borrego J, Cunningham S, Burgess KE. Retrospective evaluation of canine heart base tumours treated with toceranib phosphate (Palladia): 2011–2018. Vet Comp Oncol. 2019;17:465–71.
London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®) in solid tumours. Veterinary and Comparative Oncology. 2012;10:194–205.
Rippy SB, Gardner HL, Nguyen SM, et al. A pilot study of toceranib/vinblastine therapy for canine transitional cell carcinoma. BMC Vet Res. 2016;12:257.
Tjostheim SS, Stepien RL, Markovic LE, Stein TJ. Effects of toceranib phosphate on systolic blood pressure and proteinuria in dogs. J Vet Intern Med. 2016;30:951–7.
Lankhorst S, Kappers MHW, van Esch JHM, Danser AHJ, van den Meiracker AH. Hypertension during vascular endothelial growth factor inhibition: focus on nitric oxide, endothelin-1, and oxidative stress. Antioxid Redox Signal. 2014;20:135–45.
• Neves KB, Rios FJ, van der Mey L, Alves-Lopes R, Cameron AC, Volpe M, Montezano AC, Savoia C, Touyz RM. VEGFR (vascular endothelial growth factor receptor) inhibition induces cardiovascular damage via redox-sensitive processes. Hypertension. 2018;71:638–47 .This study shows that VEGFRi treatment of human endothelial cells in vitro decreased eNOS phosphorylation and inhibition of Nox1/4 reduced this effect. Mice treated with VEGFRi showed impaired vessel relaxation and downregulation of anti-oxidant genes. This implicates oxidative stress as an important mediator and potential target for VEGFRi toxicity..
Thijs AMJ, van Herpen CML, Sweep FCGJ, Geurts-Moespot A, Smits P, van der Graaf WTA, Rongen GA. Role of endogenous vascular endothelial growth factor in endothelium-dependent vasodilation in humans. Hypertension. 2013;61:1060–5.
Eechoute K, van der Veldt AAM, Oosting S, et al. Polymorphisms in endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF) predict sunitinib-induced hypertension. Clin Pharmacol Ther. 2012;92:503–10.
• Mirabito Colafella KM, Neves KB, Montezano AC, et al. Selective ETA vs. dual ETA/B receptor blockade for the prevention of sunitinib-induced hypertension and albuminuria in WKY rats. Cardiovascular Research. 2020;116:1779–90. This study in rats demonstrated that VEGFRi treatment induced mesenteric vessel oxidative stress, proteinuria and hypertension all of which were reversed by co-treatment with an endothelin A receptor antagonist..
Kim Y-W, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–31.
Kappers MHW, de Beer VJ, Zhou Z, Danser AHJ, Sleijfer S, Duncker DJ, van den Meiracker AH, Merkus D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59:151–7.
Kappers MHW, Smedts FMM, Horn T, van Esch JHM, Sleijfer S, Leijten F, Wesseling S, Strevens H, Jan Danser AH, van den Meiracker AH. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension. 2011;58:295–302.
Amraoui F, Spijkers L, Lahsinoui HH, Vogt L, van der Post J, Peters S, Afink G, Ris-Stalpers C, van den Born B-J. SFlt-1 elevates blood pressure by augmenting endothelin-1-mediated vasoconstriction in mice. PLOS ONE. 2014;9:e91897.
Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228:R57–71.
Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res. 2017;120:1658–73.
Kikuchi S, Yoshioka Y, Prieto-Vila M, Ochiya T. Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. Int J Mol Sci. 2019;20:2584.
• Neves KB, Rios FJ, Jones R, Evans TRJ, Montezano AC, Touyz RM. Microparticles from vascular endothelial growth factor pathway inhibitor-treated cancer patients mediate endothelial cell injury. Cardiovasc Res. 2019;115:978–88. The authors demonstrate that treatment with VEGFRi induces EC microparticle release, which is a biomarker for EC injury. They also show that the microparticles isolated from patients on VEGFRi therapy induce expression of ET-1 and reduce eNOS expression in human primary aortic endothelial cells, suggesting that EC microparticles contribute to VEGFRi-induced hypertension..
van Dorst DCH, Dobbin SJH, Neves KB, Herrmann J, Herrmann SM, Versmissen J, Mathijssen RHJ, Danser AHJ, Lang NN. Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res. 2021;128:1040–61.
Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864–86.
Catino AB, Hubbard RA, Chirinos JA, et al (2018) Longitudinal assessment of vascular function with sunitinib in patients with metastatic renal cell carcinoma. Circulation: Heart Failure 11:e004408
Dumor K, Shoemaker-Moyle M, Nistala R, Whaley-Connell A. Arterial stiffness in hypertension: an update. Curr Hypertens Rep. 2018;20:72.
Hsu P-Y, Mammadova A, Benkirane-Jessel N, Désaubry L, Nebigil CG. Updates on anticancer therapy-mediated vascular toxicity and new horizons in therapeutic strategies. Frontiers in Cardiovascular Medicine. 2021;8:726.
Steeghs N, Gelderblom H, Roodt J op’t, Christensen O, Rajagopalan P, Hovens M, Putter H, Rabelink TJ, de Koning E. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–6.
Kappers MHW, van Esch JHM, Sluiter W, Sleijfer S, Danser AHJ, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–81.
Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23.
Van Wynsberghe M, Flejeo J, Sakhi H, Ollero M, Sahali D, Izzedine H, Henique C. Nephrotoxicity of anti-angiogenic therapies. Diagnostics. 2021;11:640.
Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, Beckman JA, Harrison DG, Moslehi J. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor–associated hypertension and vascular disease. Hypertension. 2018. https://doi.org/10.1161/HYPERTENSIONAHA.117.10271.
Hirashima Y, Yamada Y, Tateishi U, Kato K, Miyake M, Akiyoshi K, Horita Y, Nagashima K, Shirao K. New analysis of the hypertension mechanism in bevacizumab-treated patients using 3-tesla dynamic contrast-enhanced magnetic resonance imaging. JCO. 2011;29:450–450.
Lankhorst S, Baelde HJ, Clahsen-van Groningen MC, Smedts FMM, Danser AHJ, van den Meiracker AH. Effect of high salt diet on blood pressure and renal damage during vascular endothelial growth factor inhibition with sunitinib. Nephrol Dial Transplant. 2016;31:914–21.
Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol. 2010. https://doi.org/10.1016/j.semnephrol.2010.09.007.
Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–36.
Seki H. Balance of antiangiogenic and angiogenic factors in the context of the etiology of preeclampsia. Acta Obstet Gynecol Scand. 2014;93:959–64.
Gatford KL, Andraweera PH, Roberts CT, Care AS. Animal models of preeclampsia: causes, consequences, and interventions. Hypertension. 2020;75:1363–81.
Verdonk K, Saleh L, Lankhorst S, et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin–angiotensin–aldosterone system suppression. Hypertension. 2015;65:1316–23.
Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–24.
Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–8.
Gal J, Milano G, Brest P, et al. VEGF-related germinal polymorphisms may identify a subgroup of breast cancer patients with favorable outcome under bevacizumab-based therapy—a message from COMET, a French Unicancer Multicentric Study. Pharmaceuticals. 2020;13:414.
Frey MK, Dao F, Olvera N, Konner JA, Dickler MN, Levine DA. Genetic predisposition to bevacizumab-induced hypertension. Gynecol Oncol. 2017;147:621–5.
Li M, Mulkey F, Jiang C, et al. Identification of a genomic region between SLC29A1 and HSP90AB1 associated with risk of bevacizumab-induced hypertension: CALGB 80405 (Alliance). Clin Cancer Res. 2018;24:4734–44.
Song Y, Xiao J, Fang W, et al. The relationship between treatment-induced hypertension and efficacy of anlotinib in recurrent or metastatic esophageal squamous cell carcinoma. Cancer Biol Med. 2021;18:562–8.
Fang S-C, Huang W, Zhang Y-M, Zhang H-T, Xie W-P. Hypertension as a predictive biomarker in patients with advanced non-small-cell lung cancer treated with apatinib. Onco Targets Ther. 2019;12:985–92.
Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity. Ann Oncol. 2007;18:1117.
Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–30.
Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Clinical course of advanced non–small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28:949–54.
Österlund P, Soveri L-M, Isoniemi H, Poussa T, Alanko T, Bono P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer. 2011;104:599–604.
George S, Reichardt P, Lechner T, Li S, Cohen DP, Demetri GD. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib ‡. Ann Oncol. 2012;23:3180–7.
Hurwitz HI, Douglas PS, Middleton JP, Sledge GW, Johnson DH, Reardon DA, Chen D, Rosen O. Analysis of early hypertension and clinical outcome with bevacizumab: results from seven phase III studies. Oncologist. 2013;18:273–80.
Morita S, Uehara K, Nakayama G, Shibata T, Oguri T, Inada-Inoue M, Shimokata T, Sugishita M, Mitsuma A, Ando Y. Association between bevacizumab-related hypertension and vascular endothelial growth factor (VEGF) gene polymorphisms in Japanese patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2013;71:405–11.
McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik O-PR, Sabbisetti VS, Bhatt RS, Simantov R, Choueiri TK. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21:2471–9.
Zhang C-J, Zhang S-Y, Zhang C-D, Lin C-R, Li X-Y, Li Q-Y, Yu H-T. Usefulness of bevacizumab-induced hypertension in patients with metastatic colorectal cancer: an updated meta-analysis. Aging (Albany NY). 2018;10:1424–41.
Montes AF, Lago NM, Rúa MC, et al. Efficacy and safety of FOLFIRI/aflibercept in second-line treatment of metastatic colorectal cancer in a real-world population: Prognostic and predictive markers. Cancer Med. 2019;8:882–9.
Duco MR, Murdock JL, Reeves DJ (2021) Vascular endothelial growth factor inhibitor induced hypertension: retrospective analysis of the impact of blood pressure elevations on outcomes. J Oncol Pharm Pract 1078155220985915
Liu Y, Zhou L, Chen Y, Liao B, Ye D, Wang K, Li H. Hypertension as a prognostic factor in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta-analysis. BMC Urol. 2019;19:49.
Hamnvik O-PR, Choueiri TK, Turchin A, McKay RR, Goyal L, Davis M, Kaymakcalan MD, Williams JS. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121:311–9.
Zhong J, Ali AN, Voloschin AD, Liu Y, Curran WJ Jr, Crocker IR, Shu H-KG. Bevacizumab-induced hypertension is a predictive marker for improved outcomes in patients with recurrent glioblastoma treated with bevacizumab. Cancer. 2015;121:1456–62.
Langenberg MHG, van Herpen CML, De Bono J, et al. Effective strategies for management of hypertension after vascular endothelial growth factor signaling inhibition therapy: results from a phase II randomized, factorial, double-blind study of cediranib in patients with advanced solid tumors. JCO. 2009;27:6152–9.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27.
Piccirillo JF. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441.
Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. JNCI. 2010;102:596–604.
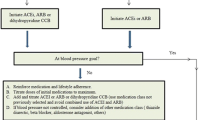
Patel S, Dushenkov A, Jungsuwadee P, Krishnaswami A, Barac A. Team-based approach to management of hypertension associated with angiogenesis inhibitors. J of Cardiovasc Trans Res. 2020;13:463–77.
Plummer C, Michael A, Shaikh G, Stewart M, Buckley L, Miles T, Ograbek A, McCormack T. Expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving bevacizumab in the UK. Br J Cancer. 2019;121:109–16.
Hassen LJ, Lenihan DJ, Baliga RR. Hypertension in the cardio-oncology clinic. Heart Fail Clin. 2019;15:487–95.
Sharma A, Burridge PW, McKeithan WL, et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med. 2017;9:eaaf2584.
Dabiré H, Dramé F, Cita N, Ghaleh B. The hypertensive effect of sorafenib is abolished by sildenafil. Cardio-Oncology. 2020;6:7.
Funding
This work was supported by grants from the NIH (CA243542 to IZJ and CL and HL155078 to IZJ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Camarda, N., Travers, R., Yang, V.K. et al. VEGF Receptor Inhibitor-Induced Hypertension: Emerging Mechanisms and Clinical Implications. Curr Oncol Rep 24, 463–474 (2022). https://doi.org/10.1007/s11912-022-01224-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01224-0