Abstract
Purpose of Review
This article reviews the current data and future directions of engineered T cell therapies in non-Hodgkin lymphomas.
Recent Findings
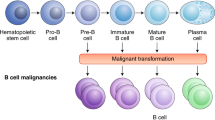
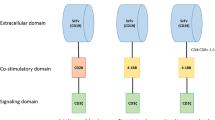
Currently, four chimeric antigen receptor (CAR) T cell products are approved: axicabtagene ciloleucel, tisagenlecleucel, lisocabtagene maraleucel, and brexucabtagene autoleucel. These products differ in construct, indication, manufacturing, clinical trial design, and toxicity profile, but all are autologous products targeting CD19. Encouraging early data is also emerging with the use of these products in additional subtypes of B cell lymphoma. Alternative engineered T cell products are also in development, including dual CD19/22 targeting CAR T cells, CD30-directed CAR T cells, allogeneic CAR T cells, and engineered natural killer (NK) cells. Preclinical data using novel CAR constructs such as cytokine-secreting CARs targeted gene delivery into the T cell receptor α constant (TRAC) locus, combination strategies, and third-generation CARs holds promise for additional novel approaches.
Summary
CAR T cells have transformed the therapeutic landscape for patients with relapsed/refractory B cell lymphomas. Early data with novel engineered cellular products is encouraging and holds promise for future clinical use.
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Sehn LH, Gascoyne RD. Diffuse large B cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22–32.
Dunleavy K, Wilson WH. Primary mediastinal B cell lymphoma and mediastinal gray zone lymphoma: do they require a unique therapeutic approach? Blood. 2015;125(1):33–9.
Casulo C, Burack WR, Friedberg JW. Transformed follicular non-Hodgkin lymphoma. Blood. 2015;125(1):40–7.
Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;1(32 31):3490–6.
van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD Study. J Clin Oncol. 2017;35(5):544–51.
Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–90.
Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–8.
Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–42.
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44.
Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52.
Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Cncol. 2016;34(11):1256–69.
Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–63.
Efficacy of axicabtagene ciloleucel compared to standard of care therapy in subjects with relapsed/refractory diffuse large B cell lymphoma.
A study to compare the efficacy and safety of JCAR017 to standard of care in adult subjects with high-risk, transplant-eligible relapsed or refractory aggressive B-cell non-Hodgkin lymphomas.
Tisagenlecleucel in adult patients with aggressive B-cell non-Hodgkin lymphoma.
Jacobson C, Chavez JC, Sehgal Ar, et al., editors. Primary analysis of Zuma-5: a phase 2 study of axicabtagene ciloleucel (Axi-Cel) in patients with relapsed/refractory (R/R) indolent non-Hodgkin Lymphoma (iNHL). 62nd American Society of Hematology Annual Meeting and Exposition; 2020 December 5–8, 2020.
Siddiqi T, Soumerai JD, Dorritie KA, et al. Rapid undetectable MRD (uMRD) responses in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) treated with lisocabtagene maraleucel (liso-cel), a CD19-directed CAR T cell product: updated results from transcend CLL 004, a phase 1/2 study including patients with high-risk disease previously treated with ibrutinib. Blood. 2019;134((Supplement_1)):503–503.
Song MK, Park BB, Uhm JE. Resistance mechanisms to CAR T-cell therapy and overcoming strategy in B-cell hematologic malignancies. Int J Mol Sci. 2019;10(20):20.
Osborne W, Marzolini M, Tholouli E, et al. Phase I Alexander study of AUTO3, the first CD19/22 dual targeting CAR T cell therapy, with pembrolizumab in patients with relapsed/refractory (r/r) DLBCL. Am J Clin Oncol. 2020;38(15_suppl):8001–8001.
Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–53.
Ramos CA, Grover NS, Beaven AW, et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2020;38(32):3794–804.
Gomes-Silva D, Srinivasan M, Sharma S, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130(3):285–96.
Maciocia PM, Wawrzyniecka PA, Philip B, et al. Targeting the T cell receptor beta-chain constant region for immunotherapy of T cell malignancies. Nat Med. 2017;23(12):1416–23.
Pinz K, Liu H, Golightly M, et al. Preclinical targeting of human T-cell malignancies using CD4-specific chimeric antigen receptor (CAR)-engineered T cells. Leuk Off J Leuk Soc Am Leuk Res Fund UK. 2016;30(3):701–7.
Chen KH, Wada M, Pinz KG, et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leuk Off J Leuk Soc Am Leuk Res Fund UK. 2017;31(10):2151–60.
Scarfo I, Ormhoj M, Frigault MJ, et al. Anti-CD37 chimeric antigen receptor T cells are active against B- and T-cell lymphomas. Blood. 2018;132(14):1495–506.
Hill LC, Rouce RH, Smith TS, et al. Safety and anti-tumor activity of CD5 CAR T-cells in patients with relapsed/refractory T-cell malignancies. Blood. 2019;134((Supplement_1)):199–199.
Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–41.
Avanzi MP, Yeku O, Li X, et al. Engineered tumor-targeted T cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep. 2018;23(7):2130–41.
Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–9.
Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847–56.
Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113–7.
Zhao Z, Condomines M, van der Stegen SJC, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28(4):415–28.
John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2(10):e26286.
Jacobson CALF, Miklos DB, Herrera AF, Westin JR, Lee J, Rossi JM, Sun J, Zheng L, Avanzi MP, Roberts ZJ. End of phase 1 results from Zuma-6: axicabtagene ciloleucel (Axi-Cel) in combination with atezolizumab for the treatment of patients with refractory diffuse large B cell lymphoma. Biol Blood Marrow Transplant. 2019;25(3):S173.
Qin JS, Johnstone TG, Baturevych A, et al. Antitumor potency of an anti-CD19 chimeric antigen receptor T-cell therapy, lisocabtagene maraleucel in combination with ibrutinib or acalabrutinib. J Immunother. 2020;43(4):107–20.
Gauthier J, Hirayama AV, Purushe J, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135(19):1650–60.
Stock S, Ubelhart R, Schubert ML, et al. Idelalisib for optimized CD19-specific chimeric antigen receptor T cells in chronic lymphocytic leukemia patients. International journal of cancer Journal international du cancer. 2019;145(5):1312–24.
Funk CR, Wang S, Waller A, et al., editors. Dual inhibition of PI3KDelta/gamma during manufacturing reprograms metabolism of CAR T cells to enhance expansion and cytotoxicity against CLL. 62nd American Society of Hematology Annual Meeting and Exposition; 2020 December 5–8, 2020.
Otahal P, Prukova D, Kral V, et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology. 2016;5(4):e1115940.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
P. Connor Johnson declares that he has no conflict of interest. JSA reports consulting for Allogene, Celgene, Juno Therapeutics, Kite Pharma, and Novartis. Jeremy S. Abramson has received compensation from AstraZeneca and Genmab for service as a consultant; has received speaker’s honoraria from Celgene and Regeneron; and has received compensation for participation on scientific advisory boards from Celgene, Juno Therapeutics, AbbVie, EMD Serono, Genentech/Roche, Janssen, Karyopharm Therapeutics, Gilead Sciences, Verastem Oncology, Bayer, Merck, Kite Pharma, Amgen, Mustang Bio, Century Therapeutics, Epizyme Seattle Genetics, Allogene Therapeutics, MorphoSys, C4 Therapeutics, Incyte Corporation, and BeiGene.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lymphomas
Rights and permissions
About this article
Cite this article
Johnson, P.C., Abramson, J.S. Engineered T Cells: CAR T Cell Therapy and Beyond. Curr Oncol Rep 24, 23–31 (2022). https://doi.org/10.1007/s11912-021-01161-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-021-01161-4