Abstract
Purpose of Review
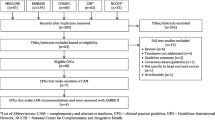
Up to 80% of patients with breast cancer are reported to use complementary and alternative medicine (CAM). Despite this high prevalence, many healthcare providers have little knowledge and education surrounding this topic and may be inadequately prepared to discuss such therapies with their patients. Given this knowledge gap, the purpose of this study was to systematically identify the quantity and assess the quality of CAM recommendations in clinical practice guidelines (CPGs) for the treatment and/or management of breast cancer.
Recent Findings
Thirty-four CPGs were deemed eligible, 5 of which mentioned CAM, and 4 of which made CAM recommendations. Eligible CPGs containing CAM recommendations were assessed with the AGREE II instrument. Scaled domain percentages from highest to lowest were (% overall, % CAM) as follows: scope and purpose (100.0%, 100.0%), editorial independence (100.0%, 100.0%), clarity of presentation (97.2%, 80.6%), rigour of development (80.2%, 80.2%), stakeholder involvement (88.9%, 77.8%), and applicability (58.3%, 58.3%).
Summary
CPGs with favourable scores may provide practitioners with guidance on safe and effective use of CAM therapies. A need exists to improve the quality of CAM recommendations in CPGs.

Similar content being viewed by others
Data Availability
All relevant data are included in this manuscript.
Abbreviations
- AGREE II:
-
Appraisal of Guidelines for Research & Evaluation II
- CAM:
-
complementary and alternative medicine
- CPG:
-
clinical practice guideline
- NCCIH:
-
National Center for Complementary and Integrative Health
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13(11):1387–97. https://doi.org/10.7150/ijbs.21635.
Leggett S, Koczwara B, Miller M. The impact of complementary and alternative medicines on cancer symptoms, treatment side effects, quality of life, and survival in women with breast cancer - a systematic review. Nutr Cancer. 2015;67(3):373–91. https://doi.org/10.1080/01635581.2015.1004731.
Waks AG, Winer EP. Breast Cancer treatment: a review. JAMA. 2019;321(3):288–300. https://doi.org/10.1001/jama.2018.19323.
Subramani R, Lakshmanaswamy R. Complementary and alternative medicine and breast cancer. Progress in molecular biology and translational science. 2017 Jan 1;151:231-74. https://doi.org/10.1016/bs.pmbts.2017.07.008.
Saquib J, Madlensky L, Kealey S, Saquib N, Natarajan L, Newman VA, et al. Classification of CAM use and its correlates in patients with early-stage breast cancer. Integr Cancer Ther. 2011;10(2):138–47. https://doi.org/10.1177/1534735410392578.
Ng JY, Boon HS, Thompson AK, Whitehead CR. Making sense of “alternative”, “complementary”, “unconventional” and “integrative” medicine: exploring the terms and meanings through a textual analysis. BMC Complement Altern Med. 2016;16(134):1. https://doi.org/10.1186/s12906-016-1111-3.
Blaes AH, Kreitzer MJ, Torkelson C, Haddad T. Nonpharmacologic complementary therapies in symptom management for breast cancer survivors. Semin Oncol. 2011;38(3):394–402. https://doi.org/10.1053/j.seminoncol.2011.03.009.
Lengacher CA, Bennett MP, Kip KE, Gonzalez L, Jacobsen P, et al. Relief of symptoms, side effects, and psychological distress through use of complementary and alternative medicine in women with breast cancer. Oncol Nurs Forum. 2006;33(1):1–9. https://doi.org/10.1188/06.ONF.
Tautz E, Momm F, Hasenburg A, Guethlin C. Use of complementary and alternative medicine in breast cancer patients and their experiences: a cross-sectional study. Eur J Cancer. 2012;48(17):3133–9. https://doi.org/10.1016/j.ejca.2012.04.021.
Boon H, Brown JB, Gavin A, Kennard MA, Stewart M. Decision to use or not to use breast cancer survivors’ perceptions of complementary/alternative medicine (CAM): making the decision to use or not to use. Qual Health Res. 1999;9(5):639–53. https://doi.org/10.1177/104973299129122135.
Tasaki K, Maskarinec G, Shumay DM, Tatsumura Y, Kakai H. Communication between physicians and cancer patients about complementary and alternative medicine: exploring patients’ perspectives. Psycho-Oncology. 2002;11:212–20. https://doi.org/10.1002/pon.552.
Brazier A. Integrative practices of Canadian oncology health professionals. Curr Oncol. 2008;15(2):87–91. https://doi.org/10.3747/co.v15i0.283.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. https://www.training.cochrane.org/handbook/current.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. https://doi.org/10.7326/0003-4819-151-4-200908180-00135.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Can Med Assoc J. 2010;182(18):E839–42. https://doi.org/10.1016/j.ypmed.2010.08.005.
Shimoi T, Nagai S, Yoshinami T, Takahashi M, Arioka H, et al. The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer. 2020;27(3):322–31. https://doi.org/10.1007/s12282-020-01085-0.
Ditsch N, Untchb M, Thillc M, Müllerd V, Janni W, et al. AGO recommendations for the diagnosis and treatment of patients with early breast cancer: update 2019. Breast Care. 2019;14(4):224–45. https://doi.org/10.1159/000501000.
Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C, et al. Radiation therapy for the whole breast: Executive Summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8(3):145–52. https://doi.org/10.1016/j.prro.2018.01.012.
Runowicz C, Leach C, Henry N, Henry K, Mackey H, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43–73. https://doi.org/10.3322/caac.21319.
National Collaborating Centre for Cancer (UK). Advanced Breast Cancer: Diagnosis and Treatment. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2009 Feb. PMID: 21901868. https://www.pubmed.ncbi.nlm.nih.gov/21901868/.
Spronk I, Korevaar JC, Schellevis FG, Albreht T, Burgers JS. Evidence-based recommendations on care for breast cancer survivors for primary care providers: a review of evidence-based breast cancer guidelines. BMJ Open. 2017;7(12). https://doi.org/10.1136/bmjopen-2016-015118This article is a review of evidence-based (EB) recommendations on survivorship care for primary care providers (PCPs) in EB breast cancer guidelines. The authors found that the number of EB recommendations in such guidelines is limited. In addition, the recommendations across guidelines varied, and most were based on low-quality evidence. Ultimately, this study concluded that higher-quality research is required to support the creating and updating of guidelines to support PCPs in providing adequate treatment for survivors of breast cancer.
Lei X, Liu F, Luo S, Sun Y, Zhu L, Su F, et al. Evaluation of guidelines regarding surgical treatment of breast cancer using the AGREE Instrument: a systematic review. BMJ Open. 2017;7(11). https://doi.org/10.1136/bmjopen-2016-014883.
Simone B, De Feo E, Nicolotti N, Ricciardi W, Boccia S. Methodological quality of English-language genetic guidelines on hereditary breast-cancer screening and management: an evaluation using the AGREE instrument. BioMed Central Med. 2012;10(143). https://doi.org/10.1186/1741-7015-10-143.
Ng JY, Liang L, Gagliardi AR. The quantity and quality of complementary and alternative medicine clinical practice guidelines on herbal medicines, acupuncture and spinal manipulation: systematic review and assessment using AGREE II. BMC Complement Altern Med. 2016;16(425):1–10. https://doi.org/10.1186/s12906-016-1410-1418.
Ng JY, Nault H, Nazir Z. Complementary and integrative medicine mention and recommendations: a systematic review and quality assessment of lung cancer clinical practice guidelines. Integr Med Res. 2020:100452. https://doi.org/10.1016/j.imr.2020.100452This article is the first to provide a systematic review of complementary and integrative medicine recommendations and conduct a quality assessment across clinical practice guidelines for the treatment and/or management of lung cancer. Finding very few recommendations overall, this study highlights a major knowledge gap, which may hinder informed shared decision-making between practitioner and provider regarding safe and effective CAM use, especially provided that most healthcare practitioners in oncology have little to no training in this topic area.
Ng JY, Sharma AE. Guidelines for cancer-related pain: a systematic review of complementary and alternative medicine recommendations. Pain Pract. 2020. https://doi.org/10.1111/papr.12964This article is the first to provide a systematic review of complementary and alternative medicine recommendations and conduct a quality assessment across clinical practice guidelines for the treatment and/or management of cancer-related pain. Finding few recommendations overall, this study highlights a major knowledge gap, which may hinder informed shared decision-making between practitioner and provider regarding safe and effective CAM use, especially provided that most healthcare practitioners in oncology have little to no training in this topic area.
Ng JY, Thakar H. Complementary and alternative medicine mention and recommendations are lacking in colon cancer clinical practice guidelines: a systematic review. Adv Integr Med. 2020. https://doi.org/10.1016/j.aimed.2020.06.002This article is the first to provide a systematic review of complementary and alternative medicine recommendations and conduct a quality assessment across clinical practice guidelines for the treatment and/or management of colon cancer. Finding no recommendations overall, this study highlights a major knowledge gap, which may hinder informed shared decision-making between practitioner and provider regarding safe and effective CAM use, especially provided that most healthcare practitioners in oncology have little to no training in this topic area.
Ng JY, Lau SK. Complementary and alternative medicine status in ovarian cancer guidelines: a systematic review. Eur J Integr Med. 2020:101227. https://doi.org/10.1016/j.eujim.2020.101227This article is the first to provide a systematic review of complementary and alternative medicine recommendations and conduct a quality assessment across clinical practice guidelines for the treatment and/or management of ovarian cancer. Finding no recommendations overall, this study highlights a major knowledge gap, which may hinder informed shared decision-making between practitioner and provider regarding safe and effective CAM use, especially provided that most healthcare practitioners in oncology have little to no training in this topic area.
Ng JY, Nazir Z, Nault H. Complementary and alternative medicine recommendations for depression: a systematic review and assessment of clinical practice guidelines. BMC Complement Med Ther. 2020;20(299):1–15. https://doi.org/10.1186/s12906-020-03085-1.
American Society of Clinical Oncology (ASCO). ASCO guidelines methodology manual [Internet]. [place unknown]: ASCO; 2020 [updated 2020 Jul 23; cited 2020 Nov 20]. Available from: https://www.asco.org/research-guidelines/quality-guidelines/guidelines-tools-resources/guideline-methodology
Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations [Internet]. The GRADE Working Group; 2013 [updated 2013 Oct; cited 2020 Nov 20]. Available from: https://www.guidelinedevelopment.org/handbook
National Institute for Health and Care Institute (NICE). Developing NICE guidelines: the manual [Internet]. [place unknown]. NICE; 2014 [updated 2020 Oct 15; cited 2020 Nov 20]. Available from: www.nice.org.uk/process/pmg20
Scottish Intercollegiate Guidelines Network (SIGN). A guideline developer’s handbook [Internet]. Edinburgh: SIGN; 2019. (SIGN publication no. 50). [updated 2019 Nov; cited 2020 Nov 20]. Available from: http://www.sign.ac.uk
Acknowledgements
JYN was awarded a research scholarship and an entrance scholarship from the Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences at McMaster University.
Author information
Authors and Affiliations
Contributions
JYN: designed and conceptualized the study, collected and analysed data, co-rafted the manuscript, and gave final approval of the version to be published.
HS: assisted with the collection and analysis of data, co-drafted the manuscript, and gave final approval of the version to be published.
SKCL: assisted with the collection and analysis of data, edited the manuscript, and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study involved a systematic review of peer-reviewed literature only; it did not require ethics approval or consent to participate.
Consent for Publication
All authors consent to this manuscript’s publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Integrative Care
Rights and permissions
About this article
Cite this article
Ng, J.Y., Sahak, H. & Lau, S.K.C. A Systematic Review and Quality Assessment of Breast Cancer Clinical Practice Guidelines Providing Complementary and Alternative Medicine Recommendations. Curr Oncol Rep 23, 112 (2021). https://doi.org/10.1007/s11912-021-01109-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01109-8