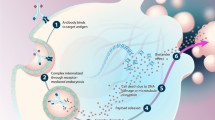
Abstract
Purpose of review
This article provides a comprehensive review of antibody-drug conjugates (ADCs) under investigation in gynecologic cancers. The structure and function of ADCs are reviewed with a focus on clinical benefit as well as toxicity profiles.
Recent findings
Several ADCs with various target antigens have been investigated in ovarian, cervical, and endometrial cancer. ADCs have consistently demonstrated favorable safety/tolerability profiles both as monotherapy and in combination therapy. In ovarian cancer, response rates have ranged from 9 to 46% for monotherapy with response rates as high as 83% in combination therapy. In patients with cervical cancer with progressive disease despite doublet therapy and bevacizumab, response rates as high as 24% have been observed.
Summary
ADCs represent a rapidly evolving field of targeted therapy which have demonstrated notable clinical benefit both as monotherapy but also in combination therapy with an overall favorable toxicity profile. With continued refinement of the target biomarkers utilized, improved clinical benefit is likely to be observed.

Similar content being viewed by others
References
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Löwenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–54. https://doi.org/10.1200/JCO.2001.19.13.3244.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. https://doi.org/10.1056/NEJMoa1209124.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28. https://doi.org/10.1056/NEJMoa1814017.
Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–21. https://doi.org/10.1056/NEJMoa1914510.
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–51. https://doi.org/10.1056/NEJMoa1814213.
Rosenberg JE, O'Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal trial of Enfortumab Vedotin in urothelial carcinoma after platinum and anti-programmed death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2019;37(29):2592–600. https://doi.org/10.1200/jco.19.01140.
Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the 'high-hanging fruit'. Nat Rev Drug Discov. 2018;17(3):197–223. https://doi.org/10.1038/nrd.2017.227.
Elias DJ, Hirschowitz L, Kline LE, Kroener JF, Dillman RO, Walker LE, et al. Phase I clinical comparative study of monoclonal antibody KS1/4 and KS1/4-methotrexate immunconjugate in patients with non-small cell lung carcinoma. Cancer Res. 1990;50(13):4154–9.
Tolcher AW, Sugarman S, Gelmon KA, Cohen R, Saleh M, Isaacs C, et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999;17(2):478–84. https://doi.org/10.1200/jco.1999.17.2.478.
Ritchie M, Tchistiakova L, Scott N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs. 2013;5(1):13–21. https://doi.org/10.4161/mabs.22854.
Hoffmann RM, Coumbe BGT, Josephs DH, Mele S, Ilieva KM, Cheung A, et al. Antibody structure and engineering considerations for the design and function of Antibody Drug Conjugates (ADCs). Oncoimmunology. 2018;7(3):e1395127. https://doi.org/10.1080/2162402x.2017.1395127.
Donaghy H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs. 2016;8(4):659–71. https://doi.org/10.1080/19420862.2016.1156829.
Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;111(6):538–49. https://doi.org/10.1093/jnci/djz035.
Lee EK, Liu JF. Antibody-drug conjugates in gynecologic malignancies. Gynecol Oncol. 2019;153(3):694–702. https://doi.org/10.1016/j.ygyno.2019.03.245.
Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70. https://doi.org/10.1158/1078-0432.CCR-04-0789.
Sun X, Ponte JF, Yoder NC, Laleau R, Coccia J, Lanieri L, et al. Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-maytansinoid conjugates. Bioconjug Chem. 2017;28(5):1371–81. https://doi.org/10.1021/acs.bioconjchem.7b00062.
Remillard S, Rebhun LI, Howie GA, Kupchan SM. Antimitotic activity of the potent tumor inhibitor maytansine. Science. 1975;189(4207):1002–5. https://doi.org/10.1126/science.1241159.
Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108(3):619–26. https://doi.org/10.1016/j.ygyno.2007.11.020.
Moore KN, Martin LP, O'Malley DM, Matulonis UA, Konner JA, Vergote I, et al. A review of mirvetuximab soravtansine in the treatment of platinum-resistant ovarian cancer. Future Oncol. 2018;14(2):123–36. https://doi.org/10.2217/fon-2017-0379.
Brown Jones M, Neuper C, Clayton A, Mariani A, Konecny G, Thomas MB, et al. Rationale for folate receptor alpha targeted therapy in "high risk" endometrial carcinomas. Int J Cancer. 2008;123(7):1699–703. https://doi.org/10.1002/ijc.23686.
Moore KN, Borghaei H, O'Malley DM, Jeong W, Seward SM, Bauer TM, et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors. Cancer. 2017;123(16):3080–7. https://doi.org/10.1002/cncr.30736.
Moore KN, Martin LP, O'Malley DM, Matulonis UA, Konner JA, Perez RP, et al. Safety and activity of Mirvetuximab Soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J Clin Oncol. 2017;35(10):1112–8. https://doi.org/10.1200/JCO.2016.69.9538.
Moore K, Matulonis U, O'Malley D, Konner J, Martin L, Perez R, et al. Mirvetuximab soravtansine (IMGN853), a folate receptor alpha (FRa)-targeting antibody-drug conjugate (ADC), in platinum-resistant epithelial ovarian cancer (EOC) patients (pts): activity and safety analyses in phase I pooled expansion cohorts. Journal of Clinical Oncology 2017. p. 5547.
Moore KN, Vergote I, Oaknin A, Colombo N, Banerjee S, Oza A, et al. FORWARD I: a phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Future Oncol. 2018;14(17):1669–78. https://doi.org/10.2217/fon-2017-0646.
Moore K, Oza A, Colombo N, Oaknin A, Scambia G, Lorusso D, et al. FORWARD I (GOG3011): a phase III study of mirvetuximab soravtansine, a folate receptor alpha (FRa)-targeting antibody-drug conjugate (ADC), versus chemotherapy in patients with platinum-resistant ovarian cancer (PROC). Ann Oncol. 2019;30:v403–34.
Moore KN, O'Malley DM, Vergote I, Martin LP, Gonzalez-Martin A, Malek K, et al. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol Oncol. 2018;151(1):46–52. https://doi.org/10.1016/j.ygyno.2018.07.017.
O'Malley DM, Matulonis UA, Birrer MJ, Castro CM, Gilbert L, Vergote I, et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2020;157:379–85. https://doi.org/10.1016/j.ygyno.2020.01.037.
Gilbert L, Oaknin A, Matulonis U, Mantia-Smaldone G, Lim P, Castro C, et al. Mirvetuximab soravtansine, a folate receptor alpha (FRa)-targeting antibody-drug conjugate (ADC), in combination with bevaciziumab in patients with platinum-agnostic ovarian cancer. J Clin Oncol. 2020;38:6004.
O'Malley D, Richardson D, Vergote I, Gilbert L, Martin L, Mantia-Smaldone G, et al. Mirvetuximab soravtaninse, a folate receptor alpha (FRa)-targeting antibody-drug conjugate (ADC), in combination with carboplatin and bevacizumab: initial results from a phase 1b study in patients with ovarian cancer. Ann Oncol. 2019:v403–34.
Matulonis U, Moore K, Martin L, Vergote I, Castro C, Gilbert L, et al. Mirvetuximab soravtaninse, a folate receptor alpha (FRalpha)-targeting antibody-drug conjugate (ADC), with pembrolizumab, in platinum-resistant ovarian cancer (PROC): Initial results of an expansion cohort from FORWARD II, a phase Ib study. Abstracts Gynaecological Cancers: Annals of Oncology; 2018.
Hassan R, Blumenschein GR Jr, Moore KN, Santin AD, Kindler HL, Nemunaitis JJ, et al. First-in-human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol. 2020;38(16):1824–35. https://doi.org/10.1200/jco.19.02085.
Bulat I, Moore K, Haceatrean A, Chung J, Rajagopalan P, Xia C, et al. Phase Ib study of anti-mesothelin antibody drug conjugate anetumab ravtansine in combination with pegylated liposomal doxorubicin in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer. J Clin Oncol. 2018;36:5571.
Coleman R, Lorusso D, Gennigens C, Gonzalez-Martin A, Randall L, Cibula D, et al. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer: results from the phase II innovaTV 204/GOG-3023/ENGOT-cx6 study. Ann Oncol. 2020:S1142–S215.
Hong DS, Concin N, Vergote I, de Bono JS, Slomovitz BM, Drew Y, et al. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clin Cancer Res. 2020;26(6):1220–8. https://doi.org/10.1158/1078-0432.ccr-19-2962.
de Bono JS, Concin N, Hong DS, Thistlethwaite FC, Machiels JP, Arkenau HT, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(3):383–93. https://doi.org/10.1016/s1470-2045(18)30859-3.
Banerjee S, Oza AM, Birrer MJ, Hamilton EP, Hasan J, Leary A, et al. Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Ann Oncol. 2018;29(4):917–23. https://doi.org/10.1093/annonc/mdy023.
Moore KN, Birrer MJ, Marsters J, Wang Y, Choi Y, Royer-Joo S, et al. Phase 1b study of anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2020;158(3):631–9. https://doi.org/10.1016/j.ygyno.2020.05.039.
Lim SI. Site-specific bioconjugation and self-assembly technologies for multi-functional biologics: on the road to the clinic. Drug Discov Today. 2020;25(1):168–76. https://doi.org/10.1016/j.drudis.2019.10.002.
Li X, Abrahams C, Zhou S, Krimm S, Henningsen R, Stephenson H, et al. Discovery and activity of STRO-002, a novel ADC targeting folate receptor alpha for ovarian and endometrial cancer. Cancer Res 2018. p. 1782.
Naumann R, Braiteh F, Diaz J, Hamilton E, Diab S, Schilder R, et al. Phase 1 dose-escalation study of STRO-002, an anti-folate receptor alpha (FRalpha) antibody drug conjugate (ADC), in patients with advanced, platinum-resistant/refractory epithelial ovarian cancer. IGSC Annual Meeting 2020.
Shimizu T, Fujiwara Y, Yonemori K, Koyama T, Shimomura A, Tamura K, et al. First-in-human (FIH) phase 1 (Ph1) study of MORAb-202 in patients (pts) with advanced folate receptor alpha (FRA)-positive solid tumors. J Clin Oncol. 2019;37:5544.
Tolcher A, Ulahannan S, Papadopoulos K, Edenfield W, Matulonis U, Burns T, et al. Phase 1 dose escalation study of XMT-1536, a novel NaPi2b-targeting antibody-drug conjugate (ADC), in patients with solid tumors likely to express NaPi2b. J Clin Oncol. 2019;37:3010.
Hamilton E, Barve M, Tolcher A, Buscema J, Papadopoulos K, Zarwan C, et al. Safety and efficacy of XMT-1536 in ovarian cancer: a subgroup analysis from the Phase I expansion study of XMT-1536, a NaPi2b antibody-drug conjugate. European Society of Medical Oncology (ESMO) Virtual Congress 2020.
Weekes CD, Lamberts LE, Borad MJ, Voortman J, McWilliams RR, Diamond JR, et al. Phase I study of DMOT4039A, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. Mol Cancer Ther. 2016;15(3):439–47. https://doi.org/10.1158/1535-7163.mct-15-0693.
Clarke J, Chu S, Siu L, Machiels J, Markman B, Heinhuis K, et al. BMS-986128, an anti-mesothelin antibody-drug conjugate (ADC), alone or in combination with nivolumab demonstrates clinical activity in patients with select advanced tumors. Molecular Cancer Therapeutics 2019. B057.
Moore K, Hamilton E, Burris H, Barroilhet L, Gutierrez M, Wang J, et al. Abstract CT036: targeting MUC16 with the THIOMABTM-drug conjugate DMUC4064A in patients with platinum-resistant ovarian cancer: a phase I expansion study. Cancer Res 2018. CT036.
Calo CA, O'Malley DM. Antibody-drug conjugates for the treatment of ovarian cancer. Expert Opin Biol Ther. 2020:1–13. https://doi.org/10.1080/14712598.2020.1776253.
Eaton JS, Miller PE, Mannis MJ, Murphy CJ. Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J Ocul Pharmacol Ther. 2015;31(10):589–604. https://doi.org/10.1089/jop.2015.0064.
Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, et al. PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2013;31(35):4400–6. https://doi.org/10.1200/jco.2013.49.7685.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David M. O'Malley has received institutional support for clinical trials from AstraZeneca, Tesaro/GlaxoSmithKline, ImmunoGen, Janssen/Johnson & Johnson, AbbVie, Regeneron, Amgen, Novocure, Genentech/Roche, VentiRx, Array BioPharma, EMD Serono, Ergomed, Ajinomoto, Inc., Ludwig Cancer Research, Stemcentrx, Inc., Cerulean Pharma, Gynecologic Oncology Group (GOG) Foundation, Bristol-Myers Squibb, Serono, Inc., TRACON Pharmaceuticals, Yale University, New Mexico Cancer Care Alliance, INC Research, Inc., inVentiv Health Clinical, Iovance Biotherapeutics, Inc., PRA International, Eisai, Agenus, Merck, Genmab, Seagen, Mersana Therapeutics, and Clovis Oncology; and has received compensation for service as a consultant/advisory board participant from AstraZeneca, Tesaro/GlaxoSmithKline, ImmunoGen, Ambry, Janssen/Johnson & Johnson, AbbVie, Regeneron, Amgen, Novocure, Genentech/Roche, GOG Foundation, Iovance Biotherapeutics, Myriad Genetics, Eisai, Agenus, Tarveda, Merck, Seagen, Novartis, Mersana Therapeutics, Clovis Oncology, Rubius Therapeutics, and Elevar Therapeutics. Corinne A. Calo declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
O’Malley, D.M., Calo, C.A. Progress in Gynecologic Cancers with Antibody Drug Conjugates. Curr Oncol Rep 23, 89 (2021). https://doi.org/10.1007/s11912-021-01080-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01080-4