Abstract
Purpose of Review
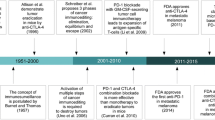
The last decade witnessed an explosion in immunotherapeutic agent approvals for various malignancies. The success of immune checkpoint inhibitors (CTLA-4 and PD-1/PD-L1) in melanoma quickly sprung to other cancer types and are considered the emerging face of oncology.
Recent Findings
Antibodies to CTLA-4 were first to enter the field, quickly followed by PD-1/PD-L1 inhibitors. Combination anti-CTLA4 and anti-PD-1/PD-L1 therapies were investigated, and after demonstrating improved responses, rapidly gained approval. Certain tumor types previously considered non-immunogenic also demonstrated durable responses which has been a remarkable discovery. However, not all tumor types respond to immunotherapies and it is widely recognized that tumor-specific immune inflammatory status predicts the best responders. Ongoing translational work indicates specific upregulation in additional immune checkpoints that circumvent response to anti-CTLA4 and anti-PD-1/PD-L1 antibodies.
Summary
Here, we provide a comprehensive review of promising therapies on the horizon with unique combinations designed to overcome resistance or expand the pool of treatment responders.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
The Nobel Prize in Physiology or Medicine 2018. NobelPrize.org. Nobel Media AB 2019. Thu. 18 Apr 2019. https://www.nobelprize.org/prizes/medicine/2018/summary/. Accessed 26 April 2019
Serrone L, et al. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. (0392–9078 (Print)).
Middleton MR, et al. Randomized Phase III Study of Temozolomide Versus Dacarbazine in the Treatment of Patients With Advanced Metastatic Malignant Melanoma. J Clin Oncol. 2000;18(1):158.
Flaherty KT, et al. Final results of E2603: A double-blind, randomized phase III trial comparing carboplatin (C)/paclitaxel (P) with or without sorafenib (S) in metastatic melanoma. J Clin Oncol. 2010;28(15_suppl):8511.
Hersh EM, et al. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann Oncol. 2015;26(11):2267–74.
Balch CM, et al. Final Version of the American Joint Committee on Cancer Staging System for Cutaneous Melanoma. J Clin Oncol. 2001;19(16):3635–48.
Alva A, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer Immunol Immunother. 2016;65(12):1533–44.
Atkins MB, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. (1081–4442 (Print)).
Hodi FS, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363(8):711–23.
Robert C, et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med. 2011;364(26):2517–26.
• Maio M, et al. Five-Year Survival Rates for Treatment-Naive Patients With Advanced Melanoma Who Received Ipilimumab Plus Dacarbazine in a Phase III Trial. J Clin Oncol. 2015;33(10):1191–6. Long-term follow-up of ipilimumab plus conventional chemotherapy demonstrating overall survival benefit.
• Schadendorf D, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015;33(17):1889–94. Long-term pooled analysis of single-agent ipilimumab with overall survival benefit.
Chapman PB, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Engl J Med. 2011;364(26):2507–16.
• Ascierto PA, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–22. High-dose vs. low-dose ipilimumab comparison demonstrating retention of efficacy but improvement in immune related adverse events.
•• Robert C, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. This was a landmark study that placed pembrolizumab as a preferred immunotherapy option for patients with cutaneous melanomas. Not only improved survival outcomes were observed, immune related adverse events were more manageable with pembrolizumab compared to ipilimumab.
•• Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–62. Long-term follow-up of single-agent pembrolizumab vs. ipilimumab that continued to show improved survival outcomes with pembrolizumab.
• Robert C, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N Engl J Med. 2015;372(4):320–30. This study placed nivolumab in the frontline setting for treatment of metastatic cutaneous melanoma.
•• Ascierto PA, et al. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 TrialNivolumab Monotherapy in Patients With Previously Untreated BRAF Wild-Type Advanced MelanomaNivolumab Monotherapy in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma. JAMA Oncol. 2019;5(2):187–94. Three-year follow-up study of nivolumab in patients with BRAF wild type cutaneous melanomas demonstrating overall survival benefit.
•• Larkin J, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. This study compared combination nivolumab plus ipilimumab and demonstrated feasibility of combination immunotherapy in the firstline treatment paradigm.
•• Hodi FS, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–92. 4-year outcome data of combination nivolumab/ ipilimumab vs. ipilimumab alone with improved survival, however treatment related toxicities were observed more frequently in the combination arm.
• Coit DG, et al. Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. 2019;17(4):367. NCCN guideliens for cutaneous melanomas provide a comprehensive guidance to oncologist to choose evidence-based treatment.
Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. (0009-921X (Print)).
Qureshi OS, et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell-Extrinsic Function of CTLA-4. Science. 2011;332(6029):600–3.
• Syn NL, et al. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–41. This study provides novel insights into mechanisms underlying de novo or acquired resistance to immune checkpoint inhibitors.
Ohigashi Y, et al. Clinical Significance of Programmed Death-1 Ligand-1 and Programmed Death-1 Ligand-2 Expression in Human Esophageal Cancer. Clin Cancer Res. 2005;11(8):2947–53.
Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236(1):219–42.
• Alsaab HO, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8(561) A comprehensive review of anti-PD1/PD-L1 inhibitors in the treatment landscape for various cancers.
Oleinika K, et al. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol. 2013;171(1):36–45.
Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20(2):241–6.
Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117(5):1167–74.
• Marin-Acevedo JA, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39. A comprehensive review of emerging treatment combinations in the immuno-oncology field.
• Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. A state-of-the-art review outlining the future of immune checkpoint therapies for various cancers.
• Maruhashi T, et al. LAG-3 inhibits the activation of CD4+ T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat Immunol. 2018;19(12):1415–26. This study explored the mechanisms of LAG-3 as an immune checkpoint inhibitor.
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492.
•• Ascierto PA, et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J Clin Oncol. 2017;35(15_suppl):9520. This early phase study of anti-LAG-3 antibody in combination with nivolumab demonstrated promising activity in cutaneous melanomas who are heavily pretreated with anti-PD-1 therapies.
• Du W, et al. TIM-3 as a Target for Cancer Immunotherapy and Mechanisms of Action. Int J Mol Sci. 2017;18(3):645. This study provides insights into the mechanism of TIM-3 as an immune checkpoint inhibitor.
Safety and Efficacy of MBG453 as Single Agent and in Combination With PDR001 in Patients With Advanced Malignancies. Available from: https://ClinicalTrials.gov/show/NCT02608268. Accessed 26 April 2019
A Phase 1 Study of TSR-022, an Anti-TIM-3 Monoclonal Antibody, in Patients With Advanced Solid Tumors (AMBER). Available from: https://ClinicalTrials.gov/show/NCT02817633. Accessed 26 April 2019
Manieri NA, Chiang EY, Grogan JL. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol. 2017;38(1):20–8.
A Study of OMP-313M32 in Subjects With Locally Advanced or Metastatic Solid Tumors. Available from: https://ClinicalTrials.gov/show/NCT03119428. Accessed 26 April 2019
Mulati K, et al. VISTA expressed in tumour cells regulates T cell function. Br J Cancer. 2019;120(1):115–27.
A Study of CA-170 (Oral PD-L1, PD-L2 and VISTA Checkpoint Antagonist) in Patients With Advanced Tumors and Lymphomas. Available from: https://ClinicalTrials.gov/show/NCT02812875. Accessed 26 April 2019
• Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res. 2016;22(14):3425–31. This study outlines the mechanism by which B7-H3 achieves immune evasion.
Powderly J, et al. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J Immunother Cancer. 2015;3(2):O8.
• Vijayan D, et al. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709. A state-of-the-art review of adenosinergic pathway and how it promotes immunosuppressive milieu in the tumor microenvironment.
• Willingham SB, et al. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti–PD-(L)1 and Anti–CTLA-4 in Preclinical Models. Cancer Immunol Res. 2018;6(10):1136–49. A preclinical study of CPI-444, an inhibitor of adenosine pathway that places it as an attractive immunotherapy target.
• Emens L, et al. Abstract CT119: CPI-444, an oral adenosine A2a receptor (A2aR) antagonist, demonstrates clinical activity in patients with advanced solid tumors. Cancer Res. 2017;77(13 Supplement):CT119. An early phase clinical study of CPI-444, an inhibitor of adenosine pathway with evidence of preliminary efficacy.
• Aspeslagh S, et al. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. A review of anti-OX-40 agonistic approach to enhance immune destruction of cancers.
• Willoughby J, et al. OX40: Structure and function – What questions remain? Mol Immunol. 2017;83:13–22. A comprehensive review of OX-40, its biology and clinical application.
Cheuk ATC, Mufti GJ, Guinn B-a. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther. 2003;11:215.
• Bartkowiak T, Curran MA. 4-1BB Agonists: Multi-Potent Potentiators of Tumor Immunity. Front Oncol. 2015:5(117). This review provides rationale for 4-1BB agonsit therapy as a promising anti-tumor immunotherapy.
• Hamid O, et al. First in human (FIH) study of an OX40 agonist monoclonal antibody (mAb) PF-04518600 (PF-8600) in adult patients (pts) with select advanced solid tumors: Preliminary safety and pharmacokinetic (PK)/pharmacodynamic results. J Clin Oncol. 2016;34(15_suppl):3079. A first-in-human study of OX40 agonist with early evidence of clinical activity.
A Phase 1 Study to Evaluate MEDI6383 Alone and in Combination With MEDI4736 in Adult Subjects With Select Advanced Solid Tumors. Available from: https://ClinicalTrials.gov/show/NCT02221960. Accessed 26 April 2019
GSK3174998 Alone or With Pembrolizumab in Subjects With Advanced Solid Tumors (ENGAGE-1). Available from: https://ClinicalTrials.gov/show/NCT02528357. Accessed 26 April 2019
Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35(4):1016–22.
A Dose Escalation and Expansion Study of TRX518 in Combination With Cyclophosphamide Plus Avelumab in Advanced Solid Tumors. Available from: https://ClinicalTrials.gov/show/NCT03861403. Accessed 26 April 2019
Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of INCAGN01876 Combined With Immune Therapies in Advanced or Metastatic Malignancies. Available from: https://ClinicalTrials.gov/show/NCT03126110. Accessed 26 April 2019
Phase I/Ib Study of GWN323 Alone and in Combination With PDR001 in Patients With Advanced Malignancies and Lymphomas. Available from: https://ClinicalTrials.gov/show/NCT02740270. Accessed 26 April 2019
An Investigational Immuno-therapy Study of Experimental Medication BMS-986156, Given by Itself or in Combination With Nivolumab in Patients With Solid Cancers or Cancers That Have Spread. Available from: https://ClinicalTrials.gov/show/NCT02598960. Accessed 26 April 2019
Study of MK-4166 and MK-4166 in Combination With Pembrolizumab (MK-3475) in Participants With Advanced Solid Tumors (MK-4166-001). Available from: https://ClinicalTrials.gov/show/NCT02132754. Accessed 26 April 2019
Elgueta R, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–72.
•• Harriet Kluger SAW, Olszanski AJ, Schuchter L, Linette GP, Garland L, Iannotti NO, Johnson M, Avsar E, Srivastava MK, Trifan OC, Edelman MJ. Phase Ib/II of CD40 agonistic antibody APX005M in combination with nivolumab (nivo) in subjects with metastatic melanoma (M) or non-small cell lung cancer (NSCLC). in 110th Annual Meeting of the American Association for Cancer Research. 2019. Atlanta, GA: AACR; 2019. Abstract nr {CT089 / 13}. This abstract was recently presented at 2019 AACR annual meeting and shows promising early clinical activity of APX005M, a novel CD40 agonist in combination with nivolumab.
Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423.
• Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231. This study provides the molecular basis of immune evasion due to gain-of-funciton β-catenin mutations in cancers.
• Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–30. This state-of-the art review provides overview of mechanisms involving tumor evasion of immunotherapies.
• Grasso CS, et al. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018;8(6):730–49. This recent study provides molecular basis of immune evasion in colorectal cancers due to ligand-independent activation of WNT canonical signaling and how it promotes immunosuppressive tumor microenvironment.
• Mariathasan S, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544. A detailed review of how TGF-β exerts its immunosuppressive phenotype.
• Germano G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552:116. This study synthesizes available evidence outlining the mechanism of immune responsiveness in tumors with deficient DNA mismatch repair pathways.
•• Le DT, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. This study led to the approval of pembrolizumab in tumors with deficient mismatch repair, this was the first tissue-agnostic approval of any drug.
•• Ott PA, et al. Abstract CT125: A personal neoantigen vaccine, NEO-PV-01, with anti-PD1 induces broad <em>de novo</em> anti-tumor immunity in patients with metastatic melanoma, NSCLC, and bladder cancer. Cancer Res. 2018;78(13 Supplement):CT125. This early phase study provides convincing evidence that a personalized neoantigen vaccine administration leads to improved responsiveness to nivolumab.
• Chesney J, et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J Clin Oncol. 2018;36(17):1658–67. This study tested the combination of oncolytic virus, T-VEC with ipilimumab in cutaneous melanomas and showed promising early evidence of activity of this combination.
•• Long GV, et al. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(15_suppl):9568. This study tested the combination of oncolytic virus (T-VEC) with pembrolizumab with impressive response rates leading to initiation of a phase III study of this novel combination (KEYNOTE-034).
Pembrolizumab With or Without Talimogene Laherparepvec or Talimogene Laherparepvec Placebo in Unresected Melanoma (KEYNOTE-034). Available from: https://ClinicalTrials.gov/show/NCT02263508. Accessed 26 April 2019
• Wang M, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–73. This review outlines the molecular mechanisms for immune evasion due to tumor microenvironment.
• Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18(9):533–48. This comprehensive review outlines the role of integrins in cancer progression and metastasis.
• Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017;17:738. This study provides a review of neovascularization in tumors that determines vascular access to chemo or immunotherapy drugs.
• Corbet C, Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer. 2017;17:577. This review outlines the mechanims by which selective tumor hypoxia promotes tumor acidosis leading to resistance to therapies.
Wojtkowiak JW, et al. Chronic Autophagy Is a Cellular Adaptation to Tumor Acidic pH Microenvironments. Cancer Res. 2012;72(16):3938–47.
Orimo A, et al. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell. 2005;121(3):335–48.
Olumi AF, et al. Carcinoma-associated Fibroblasts Direct Tumor Progression of Initiated Human Prostatic Epithelium. Cancer Res. 1999;59(19):5002–11.
Busch S, et al. TGF-beta receptor type-2 expression in cancer-associated fibroblasts regulates breast cancer cell growth and survival and is a prognostic marker in pre-menopausal breast cancer. Oncogene. 2013;34:27.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392.
Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25(2):261–7.
Butt AQ, Mills KHG. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2013;33:4623.
Baruch K, et al. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun. 2015;6:7967.
Parker KH, et al. HMGB1 Enhances Immune Suppression by Facilitating the Differentiation and Suppressive Activity of Myeloid-Derived Suppressor Cells. Cancer Res. 2014;74(20):5723–33.
Noy R, Jeffrey W. Pollard, Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41(1):49–61.
Ostuni R, et al. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36(4):229–39.
• Cannarile MA, et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5(1):53. A review of colony stimulating factor 1 receptor inhibitors and their role in inhibition of tumor associated macrophage recruitment promoting tumor destruction.
Stanley ER, Chitu V. CSF-1 Receptor Signaling in Myeloid Cells. Cold Spring Harb Perspect Biol. 2014:6(6).
• Moon YW, et al. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J Immunother Cancer. 2015;3(1):51. A comprehensive review of IDO pathway and its role in promoting an immunosuppressive mileu in tumor microenvironment.
• Toulmonde M, et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4(1):93–7. This phase 2 study of combination pembrolizumab and metronomic cyclophosphamide in soft tissue sarcomas was a negative study and provided important insignt into how IDO1/kynurenine pathway overcomes potential for responsiveness to anti-PD-1 therapy in soft tissue sarcomas.
• Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): Only an enzyme or a checkpoint controller? J Oncol Sci. 2017;3(2):52–6. This review implores the importance of IDO1/kynurenine pathway.
•• Olszanski AJ, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: Updated phase 1 results from ECHO-202/KEYNOTE-037. Ann Oncol. 2016;27(suppl_6). This was a landmark early phase study that showed impressive responses to the combination of pembrolizumab and IDO1 inhibitor, epacadostat that resulted in activation of a phase III study (ECHO-301/KEYNOTE-252).
•• Long GV, et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: Results of the phase 3 ECHO-301/KEYNOTE-252 study. J Clin Oncol. 2018;36(15_suppl):108. This negative phase III study of pembrolizumab and epacadostat created a huge buzz in the field of combination immunotherapies. The phase II study had shown promising perliminary evidence of efficacy, this study failed to demonstrate benefit in the phase III setting.
Thomas DA, Massagué J. TGF-ß directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–80.
• Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(S11):2104–17. This review outlines the somatic driver mutations in melanomas.
•• Ott PA, et al. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5(1):16. This article provides comprehensive guidelines on devising novel combination immunotherapies in clinical trials as well as preclinical models for testing the combinations. This state-of-the-art review was produced by the combination immunotherapy task force convened by the Society for Immunotherapy of Cancer (SITC).
Frederick DT, et al. BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clin Cancer Res. 2013;19(5):1225–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Babar Bashir declares that he has no conflict of interest.
Melissa A. Wilson has received compensation from Bristol-Myers Squibb for service on an advisory board.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Melanoma
Rights and permissions
About this article
Cite this article
Bashir, B., Wilson, M.A. Novel Immunotherapy Combinations. Curr Oncol Rep 21, 96 (2019). https://doi.org/10.1007/s11912-019-0851-x
Published:
DOI: https://doi.org/10.1007/s11912-019-0851-x