Abstract
Purpose of Review
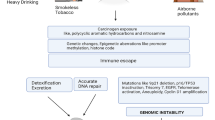
The aim of this article is to provide clinicians and pathologists with an understanding of the aetiopathology, pathogenesis and classification of vulval neoplasia and their molecular correlates.
Recent Findings
There is an increased understanding of subcellular changes in vulvar malignancies. These provide the direction for further research and aid personalised treatment for patients.
Summary
The article explores concepts of the aetiology of vulvar cancer and updates the reader with the equivalence of terminology of preneoplastic vulval disease. The differential diagnosis of squamous neoplasia and their clinicopathological correlation is detailed. The salient findings from recent literature into the understanding of the disease of squamous cell neoplasia and rare vulvar malignancies are summarised.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Weinberg D, Gomez-Martinez RA. Vulvar cancer. Obstet Gynecol Clin N Am. 2019;46(1):125–35. https://doi.org/10.1016/j.ogc.2018.09.008.
Gadducci A, Cionini L, Romanini A, Fanucchi A, Genazzani AR. Old and new perspectives in the management of high-risk, locally advanced or recurrent, and metastatic vulvar cancer. Crit Rev Oncol Hematol. 2006;60(3):227–41.
Judson PL, Habermann EB, Baxter NN, et al. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107:1018–2.
Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16(3):205–42.
Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of the female reproductive Organs, 4th edn. IARC Lyon; 2014.
Bornstein J, Bogliatto F, Haefner HK, Stockdale CK, Preti M, Bohl TG, et al. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2016;20(1):11–4.
• Hinten F, Molijn A, Eckhardt L, Massuger LFAG, Quint W, Bult P, et al. Vulvar cancer: two pathways with different localization and prognosis. Gynecol Oncol. 2018;149(2):310–7. https://doi.org/10.1016/j.ygyno.2018.03.003. A study of 318 patients showing that the HPV-associated cancers have a significantly better outcome.
McCluggage WG. Premalignant lesions of the lower female genital tract: cervix,vagina and vulva. Pathology. 2013;45(3):214–28. https://doi.org/10.1097/PAT.0b013e32835f21b1.
Michels KB, Zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–70. https://doi.org/10.1016/S0140-6736(09)61247-2.
Nooij LS, Ter Haar NT, Ruano D, Rakislova N, van Wezel T, Smit VTHBM, et al. Genomic characterization of vulvar (pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin Cancer Res. 2017;23(22):6781–678.
Kokka F, Singh N, Faruqi A, Gibbon K, Rosenthal AN. Is differentiated vulval intraepithelial neoplasia the precursor lesion of human papillomavirus-negative vulval squamous cell carcinoma? Int J Gynecol Cancer. 2011;21(7):1297–30.
• Hoang LN, Park KJ, Soslow RA, Murali R. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48(4):291–302. https://doi.org/10.1016/j.pathol.2016.02.015. A comprehensive review of classification, histomorphology and molecular genetics of both types of VIN.
Chiesa-Vottero A, Dvoretsky PM, Hart WR. Histopathologic study of thin vulvar squamous cell carcinomas and associated cutaneous lesions: a correlative study of 48 tumors in 44 patients with analysis of adjacent vulvar intraepithelial neoplasia types and lichen sclerosus. Am J Surg Pathol. 2006;30(3):310–8.
Eva LJ, Ganesan R, Chan KK, Honest H, Luesley DM. Differentiated-type vulval intraepithelial neoplasia has a high-risk association with vulval squamous cell carcinoma. Int J Gynecol Cancer. 2009;19(4):741–4.
Eva LJ, Ganesan R, Chan KK, Honest H, Malik S, Luesley DM. Vulval squamous cell carcinoma occurring on a background of differentiated vulval intraepithelial neoplasia is more likely to recur: a review of 154 cases. J Reprod Med. 2008;53(6):397–401.
• Jin C, Liang S. Differentiated vulvar intraepithelial neoplasia: a brief review of clinicopathologic features. Arch Pathol Lab Med. 2018. https://doi.org/10.5858/arpa.2018-0019-RS. This article summarises the challenges in histological diagnosis of dVIN and provides the key histologic features that aids the diagnosis.
Trietsch MD, Nooij LS, Gaarenstroom KN, van Poelgeest MI. Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursor lesions: a review of the current literature. Gynecol Oncol. 2015;136(1):143–57. https://doi.org/10.1016/j.ygyno.2014.11.002.
Zięba S, Kowalik A, Zalewski K, Rusetska N, Goryca K, Piaścik A, et al. Somatic mutation profiling of vulvar cancer: exploring therapeutic targets. Gynecol Oncol. 2018;150(3):552–61. https://doi.org/10.1016/j.ygyno.2018.06.026.
Leonard S, Pereira M, Fox R, Gordon N, Yap J, Kehoe S, et al. Over-expression of DNMT3A predicts the risk of recurrent vulvar squamous cell carcinomas. Gynecol Oncol. 2016;143(2):414–20. https://doi.org/10.1016/j.ygyno.2016.09.001.
Yap J, Fox R, Narsia N, Pinheiro-Maia S, Pounds R, Woodman C, et al. Under expression of the Sonic Hedgehog receptor,Patched1 (PTCH1), is associated with an increased risk of local recurrence in squamous cell carcinoma of the vulva arising on a background of Lichen Sclerosus. PLoS One. 2018;13(10). https://doi.org/10.1371/journal.pone.0206553.
De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–36. https://doi.org/10.1002/ijc.24116.
Muñoz N, Kjaer SK, Sigurdsson K, Iversen O-E, Hernandez-Avila M, Wheeler CM, et al. Haupt; Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325–39. https://doi.org/10.1093/jnci/djp534.
Ordi J, Alejo M, Fusté V, Lloveras B, Del Pino M, Alonso I, et al. HPV-negative vulvar intraepithelial neoplasia (VIN) with basaloid histologic pattern: an unrecognized variant of simplex (differentiated) VIN. Am J Surg Pathol. 2009;33(11):1659–65. https://doi.org/10.1097/PAS.0b013e3181b40081.
Watkins JC, Yang E, Crum CP, Herfs M, Gheit T, Tommasino M, et al. Classic vulvar intraepithelial neoplasia with superimposed lichen simplex chronicus: a unique variant mimicking differentiated vulvar intraepithelial neoplasia. Int J Gynecol Pathol. 2019;38(2):175–82. https://doi.org/10.1097/PGP.0000000000000509.
Nooij LS, Dreef EJ, Smit VT, van Poelgeest MI, Bosse T. Stathmin is a highly sensitive and specific biomarker for vulvar high-grade squamous intraepithelial lesions. J Clin Pathol. 2016;69(12):1070–5. https://doi.org/10.1136/jclinpath-2016-20367.
Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001;20:16–30.
Yang EJ, Kong CS, Longacre TA. Vulvar and anal intraepithelial neoplasia: terminology, diagnosis, and ancillary studies. Adv Anat Pathol. 2017;24(3):136–50. https://doi.org/10.1097/PAP.0000000000000149.
Reutter JC, Walters RA, Selim MA. Differentiated vulvar intraepithelial neoplasia: what criteria do we use in practice? J Low Genit Tract Dis. 2016;20(3):261–6. https://doi.org/10.1097/LGT.0000000000000211.
Hoevenaars BM, van der Avoort IA, de Wilde PC, et al. A panel of p16(INK4A), MIB1 and p53 proteins can distinguish between the 2 pathways leading to vulvar squamous cell carcinoma. Int J Cancer. 2008;123:2767–73.
Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol. 2000;24(3):429–41.
Singh N, Leen SL, Han G, Faruqi A, Kokka F, Rosenthal A, et al. Expanding the morphologic spectrum of differentiated VIN (dVIN) through detailed mapping of cases with p53 loss. Am J Surg Pathol. 2015;39(1):52–60. https://doi.org/10.1097/PAS.0000000000000291.
Liegl B, Regauer S. p53 immunostaining in lichen sclerosus is related to ischaemic stress and is not a marker of differentiated vulvar intraepithelial neoplasia (d-VIN). Histopathology. 2006;48:268–74.
Podoll MB, Singh N, Gilks CB, Moghadamfalahi M, Sanders MA. Assessment of CK17 as a marker for the diagnosis of differentiated vulvar intraepithelial neoplasia. Int J Gynecol Pathol. 2017;36(3):273–80. https://doi.org/10.1097/PGP.0000000000000317.
Dasgupta S, Ewing-Graham PC, van Kemenade FJ, van Doorn HC, Noordhoek Hegt V, Koljenović S. Differentiated vulvar intraepithelial neoplasia (dVIN): the most helpful histological features and the utility of cytokeratins 13 and 17. Virchows Arch. 2018;473(6):739–47. https://doi.org/10.1007/s00428-018-2436-8.
Nascimento AF, Granter SR, Cviko A, Yuan L, Hecht JL, Crum CP. Vulvar acanthosis with altered differentiation: a precursor to verrucous carcinoma? Am J Surg Pathol. 2004;28(5):638–43.
Watkins JC, Howitt BE, Horowitz NS, Ritterhouse LL, Dong F, MacConaill LE, et al. Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod Pathol. 2017;30(3):448–58. https://doi.org/10.1038/modpathol.2016.
Jeffreys M, Jeffus SK, Herfs M, Quick CM. Accentuated p53 staining in usual type vulvar dysplasia-a potential diagnostic pitfall. Pathol Res Pract. 2018;214(1):76–9. https://doi.org/10.1016/j.prp.2017.11.009.
Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57(1):9–30.
Carli P, Cattaneo A, De Magnis A, Biggeri A, Taddei G, Giannotti B. Squamous cell carcinoma arising in vulval lichen sclerosus: a longitudinal cohort study. Eur J Cancer Prev. 1995;4(6):491–5.
Cooper SM, Gao XH, Powell JJ, Wojnarowska F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140(6):702–6.
Bradford J, Fischer G. Long-term management of vulval lichen sclerosus in adult women. Aust N Z J Obstet Gynaecol. 2010;50(2):148–52. https://doi.org/10.1111/j.1479-828X.2010.01142.x.
van Seters M, Kate FJ, van Beurden M, et al. In the absence of (early) invasive carcinoma, vulvar intraepithelial neoplasia associated with lichen sclerosus is mainly of undifferentiated type: new insights in histology and aetiology. J Clin Pathol. 2007;60:504–9.
Gambichler T, Kammann S, Tigges C, Kobus S, Skrygan M, Meier JJ, et al. Cell cycle regulation and proliferation in lichen sclerosus. Regul Pept. 2011;167(2–3):209–14. https://doi.org/10.1016/j.regpep.2011.02.003.
Ren L, Zhao Y, Huo X, Wu X. MiR-155-5p promotes fibroblast cell proliferation and inhibits FOXO signaling pathway in vulvar lichen sclerosis by targeting FOXO3and CDKN1B. Gene. 2018;653:43–50. https://doi.org/10.1016/j.gene.2018.01.049.
Salcedo MP, Sood AK, Dos Reis R, Ramalingam P, Chen C, Frumovitz M, et al. Perineural invasion (PNI) in vulvar carcinoma: a review of 421 cases. Gynecol Oncol. 2019;152(1):101–5. https://doi.org/10.1016/j.ygyno.2018.10.035.
Rolfe KJ, Crow JC, Benjamin E, Reid WM, Maclean AB, Perrett CW. Cyclin D1 and retinoblastoma protein in vulvar cancer and adjacent lesions. Int J Gynecol Cancer. 2001;11(5):381–6.
de Melo MB, Lavorato-Rocha AM, Rodrigues IS, Baiocchi G, Cestari FM, Stiepcich MM, et al. Prognostic significance of c-KIT in vulvar cancer: bringing this molecular marker from bench to bedside. J Transl Med. 2012;10:150. https://doi.org/10.1186/1479-5876-10-150.
Dong F, Kojiro S, Borger DR, Growdon WB, Oliva E. Squamous cell carcinoma of the vulva: a subclassification of 97 cases by clinicopathologic, immunohistochemical, and molecular features (p16, p53, and EGFR). Am J Surg Pathol. 2015;39(8):1045–53. https://doi.org/10.1097/PAS.0000000000000454.
Dhakal HP, Nesland JM, Førsund M, Trope CG, Holm R. Primary tumor vascularity, HIF-1α and VEGF expression in vulvar squamous cell carcinomas: their relationships with clinicopathological characteristics and prognostic impact. BMC Cancer. 2013;13:506. https://doi.org/10.1186/1471-2407-13-506.
Lavorato-Rocha AM, Akagi EM, de Melo Maia B, Rodrigues IS, Botelho MC, Marchi FA, et al. An integrative approach uncovers biomarkers that associate with clinically relevant disease outcomes in vulvar carcinoma. Mol Cancer Res. 2016;14(8):720–9. https://doi.org/10.1158/1541-7786.MCR-15-0366.
van der Linden M, Meeuwis KA, Bulten J, Bosse T, van Poelgeest MI, de Hullu JA. Paget disease of the vulva. Crit Rev Oncol Hematol. 2016;101:60–74. https://doi.org/10.1016/j.critrevonc.2016.03.008.
Hendi A, Brodland DG, Zitelli JA. Extramammary Paget’s disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2004;51(5):767–73.
Mantovani G, Fagotti A, Franchi M, Scambia G, Garganese G. Reviewing vulvar Paget’s disease molecular bases. Looking forward to personalized target therapies: a matter of CHANGE. Int J Gynecol Cancer. 2019. https://doi.org/10.1136/ijgc-2018-000080.
Kang Z, Xu F, Zhu Y, Fu P, Zhang QA, Hu T, et al. Genetic analysis of mismatch repair genes alterations in extrammammary Paget disease. Am J Surg Pathol. 2016;40(11):1517–25.
Mert I, Semaan A, Winer I, Morris RT, Ali-Fehmi R. Vulvar/vaginal melanoma: an updated surveillance epidemiology and end results database review, comparison with cutaneous melanoma and significance of racial disparities. Int J Gynecol Cancer. 2013;23(6):1118–25. https://doi.org/10.1097/IGC.0b013e3182980ffb.
Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–6.
Hou JY, Baptiste C, Hombalegowda RB, Tergas AI, Feldman R, Jones NL, et al. Vulvar and vaginal melanoma: a unique subclass of mucosal melanoma based on a comprehensive molecular analysis of 51 cases compared with 2253 cases of nongynecologic melanoma. Cancer. 2017;123(8):1333–44. https://doi.org/10.1002/cncr.30473.
Wernham AGH, Evans M, Taniere P, Shah F, Velangi S. Molecular profiling of female genital and anorectal melanoma in a supraregional melanoma centre. Poster presented at British Association Of Dermatology Anuual Meeting. 2018.
Barbera L, Gien LT, Sutradhar R, Thomas G, Covens A, Elit L, et al. The added value of pathology review in vulvar cancer: results from a population-based cohort study. Int J Gynecol Pathol. 2017;36(2):107–10. https://doi.org/10.1097/PGP.0000000000000313.
Bhalwal AB, Nick AM, Dos Reis R, Chen CL, Munsell MF, Ramalingam P, et al. Carcinoma of the Bartholin gland: a review of 33 cases. Int J Gynecol Cancer. 2016;26(4):785–9. https://doi.org/10.1097/IGC.0000000000000656.
De Pasquale SE, McGuinness TB, Mangan CE, Husson M, Woodland MB. Adenoid cystic carcinoma of Bartholin’s gland: a review of the literature and report of a patient. Gynecol Oncol. 1996;61(1):122–5.
Felix JC, Cote RJ, Kramer EE, Saigo P, Goldman GH. Carcinomas of Bartholin’s gland. Histogenesis and the etiological role of human papillomavirus. Am J Pathol. 1993;142(3):925–33.
Luesley DM, Tristram A, Ganesan R, et al. Guidelines for the diagnosis and management of vulval carcinoma https://www.rcog.org.uk/globalassets/documents/guidelines/vulvalcancerguideline.pdf. Accessed 21 July 2019.
van Beekhuizen HJ, Auzin M, van den Einden LC, de Hullu JA, van der Velden J, Wildhagen MF, et al. Lymph node count at inguinofemoral lymphadenectomy and groin recurrences in vulvar cancer. Int J Gynecol Cancer. 2014;24(4):773–8. https://doi.org/10.1097/IGC.0000000000000125.
Butler JS, Milliken DA, Dina R, Eccles SA, Maghami SG, Jameson C, et al. Isolated groin recurrence in vulval squamous cell cancer (VSCC). The importance of node count. Eur J Gynaecol Oncol. 2010;31(5):510–3.
Burger MP, Hollema H, Emanuels AG, Krans M, Pras E, Bouma J. The importance of the groin node status for the survival of T1 and T2 vulval carcinoma patients. Gynecol Oncol. 1995;57(3):327–34.
Kirby TO, Rocconi RP, Numnum TM, Kendrick JE, Wright J, Fowler W, et al. Outcomes of stage I/II vulvar cancer patients after negative superficial inguinal lymphadenectomy. Gynecol Oncol. 2005;98(2):309–12.
de Hullu JA, van der Avoort IA, Oonk MH, van der Zee AG. Management of vulvar cancers. Eur J Surg Oncol. 2006;32(8):825–31.
Woelber L, Mahner S, Voelker K, Eulenburg CZ, Gieseking F, Choschzick M, et al. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009;29(2):545–52.
Woelber L, Eulenburg C, Choschzick M, Kruell A, Petersen C, Gieseking F, et al. Prognostic role of lymph node metastases in vulvar cancer and implications for adjuvant treatment. Int J Gynecol Cancer. 2012;22(3):503–8. https://doi.org/10.1097/IGC.0b013e31823eed4c.
de Hullu JA, Doting E, Piers DA, Hollema H, Aalders JG, Koops HS, et al. Sentinel lymph node identification with technetium-99m-labeled nanocolloid in squamous cell cancer of the vulva. J Nucl Med. 1998;39(8):1381–5.
Van der Zee AG, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26(6):884–9. https://doi.org/10.1200/JCO.2007.14.0566.
Faruqi A, Rous B. Dataset for histopathological reporting of vulval carcinomas. 2018. https://www.rcpath.org/uploads/assets/79003d03-8e27-4bf9-9732d2f3ffc5291d/g070-dataset-for-histopathological-reporting-of-vulval-carcinomas-for-publication.pdf. Accessed 21 July 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Anthony Williams declares that he has no conflict of interest.
Sheeba Syed declares that she has no conflict of interest.
Shireen Velangi has received honoraria for the preparation of vulval dermatology lectures delivered during dermatology courses throughout the UK. These courses are run by Guy’s and St. Thomas’ NHS Foundation Trust, in association with the British Association of Dermatologists, University Hospitals Birmingham and Birmingham Children’s Hospital.
Raji Ganesan declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Williams, A., Syed, S., Velangi, S. et al. New Directions in Vulvar Cancer Pathology. Curr Oncol Rep 21, 88 (2019). https://doi.org/10.1007/s11912-019-0833-z
Published:
DOI: https://doi.org/10.1007/s11912-019-0833-z