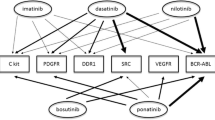
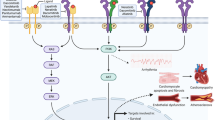
Abstract
The introduction of molecularly targeted therapies with tyrosine kinase inhibitors has revolutionized cancer therapy and has contributed to a steady decline in cancer-related mortality since the late 1990s. However, not only cardiac but also vascular toxicity has been reported for these agents, some as expected on-target effects (e.g., VEGF receptor inhibitors) and others as unanticipated events (e.g., BCR-Abl inhibitors). A sound understanding of these cardiovascular toxic effects is critical to advance mechanistic insight into vascular disease and clinical care. From a conceptual standpoint, there might be value in defining type I (permanent) and type II (transient) vascular toxicity. This review will focus on the tyrosine kinase inhibitors in current clinical use and their associated vascular side effects.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–87.
Polk A, Vistisen K, Vaage-Nilsen M, Nielsen DL. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15:47.
Doll DC, List AF, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med. 1986;105:48–51.
Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–88.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96.
Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900–2.
Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36:422–39.
Lassila M, Allen TJ, Cao Z, Thallas V, Jandeleit-Dahm KA, Candido R, et al. Imatinib attenuates diabetes-associated atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:935–42.
Aman J, van Bezu J, Damanafshan A, Huveneers S, Eringa EC, Vogel SM, et al. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation. 2012;126:2728–38.
Chislock EM, Pendergast AM. Abl family kinases regulate endothelial barrier function in vitro and in mice. PLoS One. 2013;8, e85231. This study showed for the first time that endothelial Abl is directly involved in the regulation of vascular permeability, e.g. induced by vascular endothelial growth factor, in vitro and in vivo.
Montani D, Bergot E, Gunther S, Savale L, Bergeron A, Bourdin A, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125:2128–37.
Jeon Y-W, Lee S-E, Kim S-H, Choi S-Y, Park J-E, Jeon H-R, et al. Six-year follow-up of dasatinib-related pulmonary arterial hypertension (PAH) for chronic myeloid leukemia in single center. Blood. 2013;122:4017.
Shah NP, Wallis N, Farber HW, Mauro MJ, Wolf RA, Mattei D, et al. Clinical features of pulmonary arterial hypertension in patients receiving dasatinib. Am J Hematol. 2015;90:1060–4.
Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125:901–6. This review recapitulates the vascular toxicity aspects with the BCR/ABL1 kinase inhibitors nilotinib and ponatinib and provides recommendations for clinical practice.
de Lavallade H, Punnialingam S, Milojkovic D, Bua M, Khorashad JS, Gabriel IH, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol. 2008;141:745–7.
Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27:1316–21.
Gijbels MJ, van der Cammen M, van der Laan LJ, Emeis JJ, Havekes LM, Hofker MH, et al. Progression and regression of atherosclerosis in APOE3-Leiden transgenic mice: an immunohistochemical study. Atherosclerosis. 1999;143:15–25.
Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JG, Mani V, Fallon JT, et al. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol. 2004;24:1714–9.
Sattler KJ, Galili O, Rodriguez-Porcel M, Krier JD, Lerman LO, Lerman A. Dietary reversal of experimental hypercholesterolemia improves endothelial dysfunction of epicardial arteries but not of small coronary vessels in pigs. Atherosclerosis. 2006;188:301–8.
Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33:4210–8.
Chislock EM, Ring C, Pendergast AM. Abl kinases are required for vascular function, Tie2 expression, and angiopoietin-1-mediated survival. Proc Natl Acad Sci U S A. 2013;110:12432–7. The most important study to date on the significance of Abl kinases for endothelial cells, vascular development and function. Loss of endothelial Abl contributes to vascular dysfunction, infarction, and tissue damage. Central to these phenomena is the modulation of Tie2 expression and angiopoietin-1-mediated endothelial cell survival by endothelial Abl.
Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, et al. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93:223–31.
Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation. 2010;121:898–905.
Loren CP, Aslan JE, Rigg RA, Nowak MS, Healy LD, Gruber A, et al. The BCR-ABL inhibitor ponatinib inhibits platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling, platelet activation and aggregate formation under shear. Thromb Res. 2015;135:155–60.
Herrmann J, Bell MR, Warren RL, Lerman A, Fleming MD, Patnaik M. Complicated and advanced atherosclerosis in a young woman with Philadelphia chromosome-positive acute lymphoblastic leukemia: success and challenges of BCR/ABL1-targeted cancer therapy. Mayo Clin Proc. 2015;90:1167–8.
Breccia M, Colafigli G, Molica M, Alimena G. Cardiovascular risk assessments in chronic myeloid leukemia allow identification of patients at high risk of cardiovascular events during treatment with nilotinib. Am J Hematol. 2015;90:E100–1.
Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista). Catheter Cardiovasc Interv. 2016. doi:10.1002/ccd.26379. Highly important consensus statement as first of its kind directed primarily towards the care of cancer patients with vascular disease, including vascular disease induced by cancer therapies.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–39.
Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49:287–97.
Schutz FA, Je Y, Azzi GR, Nguyen PL, Choueiri TK. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:1404–12.
Chen XL, Lei YH, Liu CF, Yang QF, Zuo PY, Liu CY, et al. Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: a meta-analysis. PLoS One. 2013;8, e66721.
Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–5.
Qi WX, Shen Z, Tang LN, Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hematol. 2014;92:71–82.
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31.
Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9:182–94.
Kappers MH, van Esch JH, Sluiter W, Sleijfer S, Danser AH, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–81.
Winnik S, Lohmann C, Siciliani G, von Lukowicz T, Kuschnerus K, Kraenkel N, et al. Systemic VEGF inhibition accelerates experimental atherosclerosis and disrupts endothelial homeostasis—implications for cardiovascular safety. Int J Cardiol. 2013;168:2453–61. Highly important study on the impact of a pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor on atherosclerosis. The key findings are a reduction in nitric oxide production and availability and progression of atherosclerotic plaques but not plaque vulnerability.
Kappers MH, Smedts FM, Horn T, van Esch JH, Sleijfer S, Leijten F, et al. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension. 2011;58:295–302.
Kappers MH, de Beer VJ, Zhou Z, Danser AH, Sleijfer S, Duncker DJ, et al. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59:151–7.
Arima Y, Oshima S, Noda K, Fukushima H, Taniguchi I, Nakamura S, et al. Sorafenib-induced acute myocardial infarction due to coronary artery spasm. J Cardiol. 2009;54:512–5.
Porto I, Leo A, Miele L, Pompili M, Landolfi R, Crea F. A case of variant angina in a patient under chronic treatment with sorafenib. Nat Rev Clin Oncol. 2010;7:476–80.
Naib T, Steingart RM, Chen CL. Sorafenib-associated multivessel coronary artery vasospasm. Herz. 2011;36:348–51.
Sen F, Yildiz I, Basaran M, Ekenel M, Oz F, Kilic L, et al. Impaired coronary flow reserve in metastatic cancer patients treated with sunitinib. J BUON. 2013;18:775–81.
Chintalgattu V, Rees ML, Culver JC, Goel A, Jiffar T, Zhang J, et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med. 2013;5:187ra69. Outstanding work that deciphered for the first time the mechanisms of sunitinib-induced cardiotoxicity, which relate to microvascular dysfunction. Central to these effects is the impairment in platelet-derived growth factor signaling and elimination of the microvascular pericyte population. These observations have far reaching implications not only for the explanation of side effects of tyrosine kinase inhibitors blocking these pathways but also the interaction of pericytes and endothelial cells of the coronary microcirculation and their link with cardiac function.
Pantaleo MA, Mandrioli A, Saponara M, Nannini M, Erente G, Lolli C, et al. Development of coronary artery stenosis in a patient with metastatic renal cell carcinoma treated with sorafenib. BMC Cancer. 2012;12:231.
Ropert S, Vignaux O, Mir O, Goldwasser F. VEGF pathway inhibition by anticancer agent sunitinib and susceptibility to atherosclerosis plaque disruption. Invest New Drugs. 2011;29:1497–9.
Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–32.
Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–41.
Herrmann J, Lerman LO, Mukhopadhyay D, Napoli C, Lerman A. Angiogenesis in atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1948–57.
Hu S, Tian J, Dong N, Sun Y, Han X, Cheng W, et al. Bevacizumab for plaque stabilization: evaluation of its effect on vasa vasorum, lipid pool, and atheroma volume by multimodality imaging techniques in an atherosclerotic rabbit model. Circulation. 2012;126, A17934.
Holm PW, Slart RH, Zeebregts CJ, Hillebrands JL, Tio RA. Atherosclerotic plaque development and instability: a dual role for VEGF. Ann Med. 2009;41:257–64.
Kus T, Aktas G, Sevinc A, Kalender ME, Camci C. Could erlotinib treatment lead to acute cardiovascular events in patients with lung adenocarcinoma after chemotherapy failure? Onco Targets Ther. 2015;8:1341–3.
Ma W, Xu M, Liu Y, Liu H, Huang J, Zhu Y, et al. Safety profile of combined therapy inhibiting EFGR and VEGF pathways in patients with advanced non-small-cell lung cancer: a meta-analysis of 15 phase II/III randomized trials. Int J Cancer. 2015;137:409–19.
Scheffel RS, Dora JM, Siqueira DR, Burttet LM, Cerski MR, Maia AL. Toxic cardiomyopathy leading to fatal acute cardiac failure related to vandetanib: a case report with histopathological analysis. Eur J Endocrinol. 2013;168:K51–4.
Kloth JS, Pagani A, Verboom MC, Malovini A, Napolitano C, Kruit WH, et al. Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer. 2015;112:1011–6.
Schreier B, Gekle M, Grossmann C. Role of epidermal growth factor receptor in vascular structure and function. Curr Opin Nephrol Hypertens. 2014;23:113–21.
Ewer MS, Suter TM, Lenihan DJ, Niculescu L, Breazna A, Demetri GD, et al. Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: a comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur J Cancer. 2014;50:2162–70.
Srikanthan A, Ethier JL, Ocana A, Seruga B, Krzyzanowska MK, Amir E. Cardiovascular toxicity of multi-tyrosine kinase inhibitors in advanced solid tumors: a population-based observational study. PLoS One. 2015;10, e0122735.
Acknowledgments
Supported, at least in part, by funding from the National Institutes of Health/National Heart, Lung, and Blood Institute (HL 1169952-03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Joerg Herrmann participated in the 2014 Ponatinib in CML Cardio-Oncology Advisory Board meeting organized by ARIAD Pharmaceuticals and is a member of the Institute for Cardio-Oncology advisory panel sponsored by Bristol-Myers Squibb.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Herrmann, J. Tyrosine Kinase Inhibitors and Vascular Toxicity: Impetus for a Classification System?. Curr Oncol Rep 18, 33 (2016). https://doi.org/10.1007/s11912-016-0514-0
Published:
DOI: https://doi.org/10.1007/s11912-016-0514-0