Abstract
Purpose of Review
Migraine is one of the top reasons for consulting a pediatric neurologist. Although the majority of children and adolescents who receive evidence-based first-line interventions for migraine will improve substantially, a subset of patients develop resistant or refractory migraine.
Recent Findings
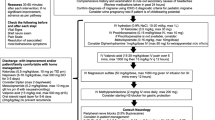
In this review, we summarize the level of evidence for a variety of acute and preventive treatment options to consider in children and adolescents with resistant or refractory migraine. We describe the level of evidence for interventional procedures (onabotulinumtoxinA injections, greater occipital and other nerve blocks), neuromodulation (single-pulse transcranial magnetic stimulation, external trigeminal nerve stimulation, remote electrical neuromodulation, and non-invasive vagal nerve stimulation), calcitonin gene-related peptide (CGRP) pathway antagonists (anti-CGRP monoclonal antibodies and gepants), psychological therapies, and manual therapies (acupuncture, craniosacral therapy, massage and physical therapy, and spinal manipulation).

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Curless RG. Diagnostic problems in three pediatric neurology practice plans. Pediatr Neurol. 1998;19(4):272–4.
Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population-based studies. Dev Med Child Neurol. 2010;52(12):1088–97.
Hershey AD, Powers SW, Lecates S, Kabbouche MA, Maynard MK. PedMIDAS: Development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034–9.
Hershey AD, Powers SW, Vockell A, LeCates S, Segers A, Kabbouche M. Development of a patient-based grading scale for PedMIDAS. Cephalalgia. 2004;24(10):844–9.
Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877–97.
•• Powers SW, Coffey CS, Chamberlin LA, Ecklund DJ, Klingner EA, Yankey JW, et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. NEJM. 2017;376(2):115. (Seminal pediatric migraine trial that showed no efficacy difference between the two most commonly prescribed preventive medications and placebo••)
•• Powers SW, Coffey CS, Chamberlin LA, Ecklund DJ, Klingner EA, Yankey JW, et al. Prevalence of headache days and disability 3 years after participation in the Childhood and Adolescent Migraine Prevention Medication Trial. JAMA Netw Open. 2021; 10.10:1–14. CHAMP follow-up study that showed that most improvements seen after pill-based treatments are sustained long-term in children and adolescents with migraine
Sacco S, Braschinsky M, Ducros A, Lampl C, Little P, Van Den BAM, et al. European Headache Federation consensus on the definition of resistant and refractory migraine developed with the endorsement of the European Migraine & Headache Alliance (EMHA). J Headache Pain. 2020;21(1):1–12.
Binder WJ, Brin MF, Blitzer A, Pogoda JM. Botulinum toxin type a (BOTOX) for treatment of migraine. Vol. 48, Disease-a-Month. 2002. p. 323–35.
Aurora SK, Aurora, Sheena K, Paul, Freeman, Marshall C, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Vol. 51, Headache. United States; 2011. p. 1358–73.
Herd CP, Tomlinson CL, Rick C, Scotton WJ, Edwards J, Ives NJ, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9(7):e027953.
Barad M, Ailani J, Hakim SM, Kissoon NR, Schuster NM. Percutaneous interventional strategies for migraine prevention: a systematic review and practice guideline. Pain Med. 2022;23(1):164–88.
Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50(9):1406–18.
Marcelo R, Freund B. The efficacy of botulinum toxin in pediatric chronic migraine: a literature review. J Child Neurol. 2020;35(12):844–51.
• Winner PK, Kabbouche M, Yonker M, Wangsadipura V, Lum A, Brin MF. A randomized trial to evaluate onabotulinumtoxinA for prevention of headaches in adolescents with chronic migraine. Headache. 2020;60(3):564–75. OnabotulinumtoxinA parallel-group randomized controlled trial in children and adolescents
• Shah S, Calderon MD, Crain N, Pham J, Rinehart J. Effectiveness of onabotulinumtoxinA (BOTOX) in pediatric patients experiencing migraines: a randomized, double-blinded, placebo-controlled crossover study in the pediatric pain population. Reg Anesth Pain Med. 2021;46(1):41–8. OnabotulinumtoxinA cross-over randomized controlled trial in children and adolescents
Szperka CL, Gelfand AA, Hershey AD. Patterns of use of peripheral nerve blocks and trigger point injections for pediatric headache: results of a survey of the American Headache Society Pediatric and Adolescent Section. Headache. 2016;56(10):1597–607.
Dubrovsky AS. Nerve blocks in paediatric and adolescent headache disorders. Curr Pain Headache Rep. 2017;21(50):1–6.
Gelfand AA, Reider ACGP. Outcomes of greater occipital nerve injections in pediatric patients with chronic primary headache disorders. Pediatr Neurol. 2014;50(2):135–9.
Lacey D. Efficacy of greater occipital nerve blocks (ONBs) in the treatment of chronic daily headaches (CDH) in adolescent females with fibromyalgia. Headache. 2008;24(3):160–77.
Renaudon-Smith E, Toolis C, Goadsby P, Prabhakar P. Greater occipital nerve injection (GONI) for chronic headache in children. J Headache Pain. 2010;11(S1):1–150.
Puledda F, Goadsby PJ, Prabhakar P. Treatment of disabling headache with greater occipital nerve injections in a large population of childhood and adolescent patients: a service evaluation. J Headache Pain. 2018;19:1.
Esparham A, Boorigie M, Ablatt S, Connelly M, Bickel J. Improving acute treatment of pediatric primary headache disorders with a novel headache treatment center: retrospective review of preliminary outcomes. J Child Neurol. 2021;36(1):54–9.
Mousa MA, Aria DJ, Mousa AA, Schaefer CM, Temkit MH, Towbin RB. Sphenopalatine ganglion nerve block for the treatment of migraine headaches in the pediatric population. Pain Physician. 2021;24(1):E111–6.
Kouri M, Somaini M, Cárdenas VHG, Niburski K, Vigouroux M, Ingelmo P. Transnasal sphenopalatine ganglion block for the preventive treatment of chronic daily headache in adolescents. Children. 2021;8(7):1–8.
Barker T. An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8(1):26–37.
Brighina F, Raieli V, Messina LM, Santangelo G, Puma D, Drago F, et al. Non-invasive brain stimulation in pediatric migraine: a perspective from evidence in adult migraine. Front Neurol. 2019;10(APR):1–5.
Beaulieu LD, Schneider C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: a literature review on parameters of application and afferents recruitment. Neurophysiol Clinlinique. 2015;45(3):223–37.
Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain spinal cord roots and peripheral nerves basic principles and procedures for routine clinical and research application An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–107.
Gilbert DL, Garvey MA, Bansal AS, Lipps T, Zhang J, Wassermann EM. Should transcranial magnetic stimulation research in children be considered minimal risk? Clin Neurophysiol. 2004;115(8):1730–9.
Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimuls. 2015;8(1):76–87.
Zewdie E, Ciechanski P, Kuo HC, Giuffre A, Kahl C, King R, et al. Safety and tolerability of transcranial magnetic and direct current stimulation in children: prospective single center evidence from 3.5 million stimulations. Brain Stimul. 2020;13(3):565–75.
• Irwin SL, Qubty W, Allen IE, Patniyot I, Goadsby PJ, Gelfand AA. Transcranial magnetic stimulation for migraine prevention in adolescents: a pilot open-label study. Headache. 2018;58(5):724–31. Transcranial magnetic stimulation open-label pilot study among children and adolescents with migraine
Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9(4):373–80.
Starling AJ, Tepper SJ, Marmura MJ, Shamim EA, Robbins MS, Hindiyeh N, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia. 2018;38(6):1038–48.
Schoenen JE, Vandersmissen B, Herroelen L, Vandenheede M, Gerard P, Magis D. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697–704.
Chou DE, Shnayderman Yugrakh M, Winegarner D, Rowe V, Kuruvilla D, Schoenen J. Acute migraine therapy with external trigeminal neurostimulation (ACME): a randomized controlled trial. Cephalalgia. 2019;39(1):3–14.
Hokenek NM, Erdogan MO, Hokenek UD, Algin A, Tekyol D, Seyhan AU. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: a randomized controlled trial. Am J Emerg Med. 2021;39:80–5.
Kuruvilla DE, Mann JI, Tepper SJ, Starling AJ, Panza G, Johnson MAL. Phase 3 randomized, double-blind, sham-controlled Trial of e-TNS for the Acute treatment of Migraine (TEAM). Sci Rep. 2022;12(1):1–11.
Vikelis M, Dermitzakis EV, Spingos KC, Vasiliadis GG, Vlachos GS, Kararizou E. Clinical experience with transcutaneous supraorbital nerve stimulation in patients with refractory migraine or with migraine and intolerance to topiramate: a prospective exploratory clinical study. BMC Neurol. 2017;17(1):1–7.
Jiang L, Yuan D, Li M, Liu C, Liu Q, Zhang Y, et al. Combination of flunarizine and transcutaneous supraorbital neurostimulation improves migraine prophylaxis. Act Neurol Scand. 2019;139(3). https://doi.org/10.1111/ane.13050
Magis D, Magis, Delphine, Sava, Simona, d’Elia, et al. Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly device in headache treatment: a survey of 2,313 headache sufferers in the general population. Vol. 14, Journal of Headache & Pain. Italy; 2013. p. 95-.
Di Fiore P, Bussone G, Galli A, Didier H, Peccarisi C, D’Amico D, et al. Transcutaneous supraorbital neurostimulation for the prevention of chronic migraine: a prospective, open-label preliminary trial. Neurol Sci. 2017;38:201–6.
Yarnitsky D, Dodick DW, Grosberg BM, Burstein R, Ironi A, Harris D, et al. Remote electrical neuromodulation (REN) relieves acute migraine: a randomized, double-blind, placebo-controlled, multicenter trial. Headache. 2019;59(8):1240–52.
•• Hershey AD, Lin T, Gruper Y, Harris D, Ironi A, Thomas M, et al. Remote electrical neuromodulation for acute treatment of migraine in adolescents. Headache. 2021;61(2):310–7. Remote electrical neuromodulation open-label trial study among children and adolescents with migraine
Yarnitsky D, Volokh L, Ironi A, Weller B, Shor M, Shifrin A, et al. Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology. 2017;88(13):1250–5.
Marmura MJ, Lin T, Harris D, Ironi A, Rosen NL. Incorporating remote electrical neuromodulation (REN) into usual care reduces acute migraine medication use: an open-label extension study. Front Neurol. 2020;11(April):1–8.
Buse D, Rabany L, Lin T, Ironi A, Connelly M, Bickel J. Combining guided intervention of education and relaxation (GIER) with remote electrical neuromodulation (REN) in the acute treatment of migraine. Pain Med. 2022. https://doi.org/10.1093/pm/pnac021.
Vecchio E, Bassez I, Ricci K, Tassorelli C, Liebler E, de Tommaso M. Effect of non-invasive vagus nerve stimulation on resting-state electroencephalography and laser-evoked potentials in migraine patients: mechanistic insights. Front Hum Neurosci. 2018. https://doi.org/10.3389/fnhum.2018.00366.
• Grazzi L, Egeo G, Liebler E, Padovan A, Barbanti P. Non-invasive vagus nerve stimulation (nVNS) as symptomatic treatment of migraine in young patients: a preliminary safety study. Neurol Sci. 2017;38(S1):S197–99. Non-invasive vagal nerve stimulation open-label pilot study among children and adolescents with migraine
Tassorelli C, Grazzi L, De Tommaso M, Pierangeli G, Martelletti P, Rainero I, et al. Noninvasive vagus nerve stimulation as acute therapy for migraine: the randomized PRESTO study. Neurology. 2018;91(4):e364–73.
Diener HC, Goadsby PJ, Ashina M, Al-Karagholi MAM, Sinclair A, Mitsikostas D, et al. Non-invasive vagus nerve stimulation (nVNS) for the preventive treatment of episodic migraine: the multicentre, double-blind, randomised, sham-controlled PREMIUM trial. Cephalalgia. 2019;39(12):1475–87.
Najib U, Smith T, Hindiyeh N, Saper J, Nye B, Ashina S, et al. Non-invasive vagus nerve stimulation for prevention of migraine: the multicenter, randomized, double-blind, sham-controlled PREMIUM II trial. Cephalalgia. 2022;033310242110688.
Lai YH, Huang YC, Huang LT, Chen RM, Chen C. Cervical noninvasive vagus nerve stimulation for migraine and cluster headache: a systematic review and meta-analysis. Neuromodulation. 2020;23(6):721–31.
Edvinsson L, Haanes KA, Warfvinge K, DiN Krause. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338–50.
Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–37.
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8.
Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine a randomized clinical trial. JAMA. 2018;319(19):1999–2008.
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–54.
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. NEJM. 2017;377(22):2123–32.
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–7.
Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Fremanezumab for the preventive treatment of chronic migraine. NEJM. 2017;377(22):2113–22.
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):E2211–21.
Dodick DW, Lipton RB, Silberstein S, Goadsby PJ, Biondi D, Hirman J, et al. Eptinezumab for prevention of chronic migraine: a randomized phase 2b clinical trial. Cephalalgia. 2019;39(9):1075–85.
Ashina M, Saper J, Cady R, Schaeffler BA, Biondi DM, Hirman J, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241–54.
•• Greene KA, Gentile CP, Szperka CL, Yonker M, Gelfand AA, Grimes B, et al. Calcitonin gene–related peptide monoclonal antibody use for the preventive treatment of refractory headache disorders in adolescents. Pediatr Neurol. 2021;114:62–7. Anti-CGRP monoclonal antibody open-label retrospective observational study among adolescents with migraine
Suleman S, Chow C, Rastogi RG. Use of anti-calcitonin gene-related peptide monoclonal antibodies in pediatric migraine prevention (4082). Neurology. 2021;96(15 Supplement):4082.
•• Szperka CL, VanderPluym J, Orr SL, Oakley CB, Qubty W, Patniyot I, et al. Recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache. 2018;58(10):1658–69. Anti-CGRP monoclonal antibody expert consensus guidelines on when to use the antibodies off-label in pediatric patients
Dodick D, Lipton R, Ailani J, Lu K, Finnegan M, Trugman J, et al. Ubrogepant for the treatment of migraine. NEJM. 2019;381:2230–41.
Lipton RB, Dodick DW, Ailani J, Lu K, Finnegan M, Szegedi A, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322(19):1887–98.
Gao B, Yang Y, Wang Z, Sun Y, Chen Z, Zhu Y, et al. Efficacy and safety of rimegepant for the acute treatment of migraine: evidence from randomized controlled trials. Front Pharmacol. 2020;10:https://doi.org/10.3389/fphar.2019.01577.
Croop R, Lipton RB, Kudrow D, Stock DA, Kamen L, Conway CM, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10268):51–60.
Goadsby PJ, Dodick DW, Ailani J, Trugman JM, Finnegan M, Lu K, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19(9):727–37.
Knestrick KE, Gibler RC, Reidy BL, Powers SW. Psychological interventions for pediatric headache disorders: a 2021 update on research progress and needs. Curr Pain Headache Rep. 2022;26(1):85–91.
•• Fisher E, Law E, Dudeney J, Palermo T, Stewart G, Eccleston C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2018;1–123. Cochrane systematic review on the efficacy of psychological interventions for the management of pediatric chronic pain including migraine
•• Powers SW, Kashikar-Zuck SM, Allen JR, LeCates SL, Slater SK, Zafar M, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA. 2013;310(24):2622–30. Seminal cognitive behavioral therapy trial for adjunctive treatment in children and adolescents with chronic migraine
Vasiliou VS, Karademas EC, Christou Y, Papacostas S, Karekla M. Acceptance and commitment therapy for primary headache sufferers: a randomized controlled trial of efficacy. J Pain. 2021;22(2):143–60.
Pielech M, Vowles KE, Wicksell R. Acceptance and commitment therapy for pediatric chronic pain: theory and application. Children. 2017;4(2):1–12.
Odell S, Logan DE. Pediatric pain management: the multidisciplinary approach. J Pain Res. 2013;6:785–90.
• Benore E, Webster EE, Wang L, Banez G. Longitudinal analysis of patient-reported outcomes from an interdisciplinary pediatric pain rehabilitation program for children with chronic migraine and headache. Headache. 2018;58(10):1556–67. Observational study on the efficacy of interdisciplinary intensive pain rehabilitation in children with migraine and other headache disorders
• Shulman J, Conroy C, Cybulski A, Smith KR, Jervis K, Johnson H, et al. Does intensive interdisciplinary pain treatment improve pediatric headache-related disability? Disabil Rehabil. 2022;44(2):194–201. Observational study on the efficacy of interdisciplinary intensive pain rehabilitation in children with migraine and other headache disorders
Hechler T, Kanstrup M, Holley AL, Simons LE, Wicksell R, Hirschfeld G, et al. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. Pediatrics. 2015;136(1):115–27.
Bethell C, Kemper KJ, Gombojav N, Koch TK. Complementary and conventional medicine use among youth with recurrent headaches. Pediatrics. 2013;132(5):e1173–83.
Dalla Libera D, Colombo B, Pavan G, Comi G. Prescription of complementary and alternative medicine (CAM) in an Italian cohort of pediatric headache patients. Neurology. 2014;35(S1):145–8.
Lu T, Lu C, Li M, Ke L, Cai H, Yang K. Reporting and methodological quality of meta-analyses of acupuncture for patients with migraine: a methodological investigation with evidence map. J Integr Med. 2022. 10.10:1–8
Linde K, Allais G, Brinkhaus B, Mehring M, Vertosick E, Vickers A, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:CD001218.
Haller H, Lauche R, Sundberg T, Dobos G, Cramer H. Craniosacral therapy for chronic pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2019;21(1):1–14.
Falsiroli Maistrello L, Rafanelli M, Turolla A. Manual therapy and quality of life in people with headache: systematic review and meta-analysis of randomized controlled trials. Curr Pain Headache Rep. 2019;23(10):23–5.
Jung A, Eschke RC, Gabler T, Pawlowsky V, Luedtke K. Effectiveness of physiotherapeutic treatment interventions on pain intensity, duration, frequency, and quality of life of patients with migraine: a systematic review. Schmerz. 2021. 10.100:1–11
Rist PM, Hernandez A, Bernstein C, Kowalski M, Osypiuk K, Vining R, et al. The impact of spinal manipulation on migraine pain and disability: a systematic review and meta-analysis. Headache. 2019;59(4):532–42.
Lawler SP, Cameron LD. A randomized, controlled trial of massage therapy as a treatment for migraine. Ann Behav Med. 2006;32(1):50–9.
Happe S, Peikert A, Siegert R, Evers S. The efficacy of lymphatic drainage and traditional massage in the prophylaxis of migraine: a randomized, controlled parallel group study. Neurol Sci. 2016;37(10):1627–32.
Chatchawan U, Eungpinichpong W, Sooktho S, Tiamkao S, Yamauchi J. Effects of Thai traditional massage on pressure pain threshold and headache intensity in patients with chronic tension-type and migraine headaches. J Altern Complement Med. 2014;20(6):486–92.
• Gottschling S, Meyer S, Gribova I, Distler L, Berrang J, Gortner L, et al. Laser acupuncture in children with headache: a double-blind, randomized, bicenter, placebo-controlled trial. Pain. 2008;137(2):405–12. Randomized controlled trial on the use of laser acupuncture for children and adolescents with headache disorders
Adams D, Cheng F, Jou H, Aung S, Yasui Y, Vohra S. The safety of pediatric acupuncture: a systematic review. Pediatrics. 2011;128:6.
Todd A, Carroll M, Robinson A, Mitchell E. Adverse events due to chiropractice and other manual therapies for infants and children: a review of the literature. J Manip Physiol Ther. 2015;38(9):699–712.
Karkhaneh M, Zorzela L, Jou H, Funabashi M, Dryden T, Vohra S. Adverse events associated with paediatric massage therapy: a systematic review. BMJ Paediatr Open. 2020;4:1.
Fisher E, Villanueva G, Henschke N, Nevitt SJ, Zempsky W, Probyn K, et al. Efficacy and safety of pharmacological, physical, and psychological interventions for the management of chronic pain in children: a WHO systematic review and meta-analysis. Pain. 2022;163(1):E1-19.
Vohra S, Johnston BC, Cramer K, Humphreys K. Adverse events associated with pediatric spinal manipulation: a systematic review. Pediatrics. 2007;119(1):275–83.
Gladstein J, Szperka C, Gelfand A. Pediatric headache. 1st ed. Cambridge, MA, US: Elsevier; 2021. p. 346.
•• Locher C, Kossowsky J, Koechlin H, Lam TL, Barthel J, Berde CB, et al. Efficacy, safety, and acceptability of pharmacologic treatments for pediatric migraine prophylaxis: a systematic review and network meta-analysis. JAMA Pediatr. 2020;174(4):341–9. (Network meta-analysis examining the efficacy and safety of pill-based preventive interventions for children and adolescents with episodic migraine)
Hornik C, Gelfand A, Szperka C, Pezzuto T, Utevsky A, Kessel S, et al. Development of a prospective real-world data clinical registry of children and adolescents with migraine. Headache. 2020;60(2):405–15.
Kommineni M, Finkel AG. Teaching headache in America: survey of neurology chairs and residency directors. Headache. 2005;45(7):862–5.
Pace A, Orr SL, Rosen NL, Safdieh JE, Bosmenier G, Sprouse-Blum AS. The current state of headache medicine education in the United States and Canada: an observational, survey-based study of neurology clerkship directors and curriculum deans. Headache. 2021;61:854–62.
Robbins MS, Rosen NL. Headache interest in academic neurology leadership: a cross-sectional study. Headache. 2018;58(1):102–8.
Gelfand AA, Qubty W, Patniyot I, Grimes B, Pletcher MJ, Goadbsy PJ, et al. Home-based trials in adolescent migraine: a randomized clinical trial. JAMA Neurol. 2017;74(6):744–5.
Shapiro RE. What will it take to move the needle for headache disorders? An advocacy perspective Headache. 2020;60(9):2059–77.
Funding
This research was supported by the Alberta Children’s Hospital Research Institute and the Department of Pediatrics, Cumming School of Medicine, University of Calgary. The Alberta Children’s Hospital Research Institute and the Department of Pediatrics provided funding for this project but had no role in design or execution of the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alison Marshall: none.
Rebecca Lindsay: none.
Michelle A. Clementi: has research funding from the Ludeman Family Center for Women’s Health Research at the University of Colorado Anschutz Medical Campus and from NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Amy A. Gelfand: in the last 24 months, Dr. Gelfand has received honoraria from UpToDate (for authorship), and stipends from JAMA Neurology for editorial work (last in July 2020) and from the American Headache Society for her role as Editor of Headache. She received grant support from the Duke Clinical Research Institute and the UCSF Resource Allocation Program. Her spouse reports research support (to UCSF) from Genentech for a clinical trial, honoraria for editorial work from Dynamed Plus, and personal compensation for medical-legal consulting.
Serena L. Orr receives royalties from Cambridge University Press. She serves on the editorial boards of Headache and Neurology. She also has research funding from the Canadian Institutes of Health Research and the Alberta Children’s Hospital Research Institute.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Headache
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marshall, A., Lindsay, R., Clementi, M.A. et al. Outpatient Approach to Resistant and Refractory Migraine in Children and Adolescents: a Narrative Review. Curr Neurol Neurosci Rep 22, 611–624 (2022). https://doi.org/10.1007/s11910-022-01224-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01224-4