Abstract
Purpose of Review
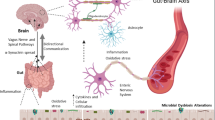
Defective gut-brain communication has recently been proposed as a promoter of neurodegeneration, but mechanisms mediating communication remain elusive. In particular, the Parkinson’s disease (PD) phenotype has been associated with both dysbiosis of intestinal microbiota and neuroinflammation. Here, we review recent advances in the PD field that connect these two concepts, providing an explanation based on enteroendocrine signaling from the gut to the brain.
Recent Findings
There have been several recent accounts highlighting the importance of the microbiota-gut-brain axis in PD. The objective of this review is to discuss the role of the neuroendocrine system in gut-brain communication as it relates to PD pathogenesis, as this system has not been comprehensively considered in prior reviews. The incretin hormone glucagon-like peptide 1 (GLP-1) is secreted by enteroendocrine cells of the intestinal epithelium, and there is evidence that it is neuroprotective in animal models and human subjects with PD. Agonists of GLP-1 receptors used in diabetes appear to be useful for preventing neurodegeneration. New tools and models have enabled us to study regulation of GLP-1 secretion by intestinal microbiota, to understand how this process may be defective in PD, and to develop methods for therapeutically modifying disease development or progression using the enteroendocrine system.
Summary
GLP-1 secretion by enteroendocrine cells may be a key mediator of neuroprotection in PD, and new findings in this field may offer unique insights into PD pathogenesis and therapeutic strategies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21.
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912.
Dodiya HB, Forsyth CB, Voigt RM, Engen PA, Patel J, Shaikh M, et al. Chronic stress-induced gut dysfunction exacerbates Parkinson's disease phenotype and pathology in a rotenone-induced mouse model of Parkinson's disease. Neurobiol Dis. 2020;135:104352.
Jackson A, Forsyth CB, Shaikh M, Voigt RM, Engen PA, Ramirez V, et al. Diet in Parkinson’s disease: critical role for the microbiome. Front Neurol. 2019;10(December):1–21.
Keshavarzian A, Engen P, Bonvegna S, Cilia R. The gut microbiome in Parkinson’s disease: a culprit or a bystander? Prog Brain Res. 2020;252:357–450.
Chen H, Ritz B. The search for environmental causes of Parkinson’s disease: moving forward. J Parkinsons Dis. 2018;8(s1):S9–17.
Manfready RA. Probing yeast for insights into neurodegenerative disease: ORFeome-wide screens for genetic modifiers of [alpha]-synuclein cytotoxicity. Biotechnol Mol Biol Rev. 2010;5(4):67–70.
Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RAE, et al. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord. 2014;29(8):999–1009.
Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14(6):625–39.
Lin CH, Chen CC, Chiang HL, Liou JM, Chang CM, Lu TP, et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflamm. 2019;16(1):129.
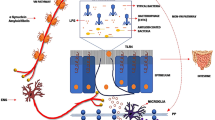
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167:1469–80.
Li Y, Perry TA, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106(4):1285–90.
• Manfready RA, Engen PA, VerhagenMetman L, Sanzo G, Goetz CG, Hall DA, et al. Attenuated postprandial GLP-1 response in Parkinson’s disease. Front Neurosci. 2021;15:660942. This study demonstrates that postprandial systemic GLP-1 is diminished in PD patients compared to household controls, suggesting that secretion may be impaired in PD.
Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110(5):517–36.
Liddle RA. Parkinson’s disease from the gut. Brain Res. 2018;1693(Pt B):201–6.
Kujawska M, Jodynis-Liebert J. What is the evidence that Parkinson's disease is a prion disorder, which originates in the gut? Int J Mol Sci. 2018;19:3573.
Borghammer P. How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord. 2018;33(1):48–57.
Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27(6):709–15.
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, et al. Colonic bacterial composition in Parkinson's disease. Mov Disord. 2015;30(10):1351–60.
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord.2016;32:66–72.
Joers V, Masilamoni G, Kempf D, Weiss AR, Rotterman TM, Murray B, et al. Microglia, inflammation and gut microbiota responses in a progressive monkey model of Parkinson's disease: a case series. Neurobiol Dis. 2020;144:105027.
Dutta G, Zhang P, Liu B. The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol. 2008;22(5):453–64.
Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK. Microbiome-microglia connections via the gut-brain axis. J Exp Med. 2019;216(1):41–59.
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–45.
Morris G, Berk M, Carvalho A, Caso JR, Sanz Y, Walder K, et al. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol Neurobiol. 2017;54(6):4432–51.
Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63.
Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). 2020;11:25.
Bolognini D, Tobin AB, Milligan G, Moss CE. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol. 2016;89(3):388–98.
• Ducastel S, Touche V, Trabelsi MS, Boulinguiez A, Butruille L, Nawrot M, et al. The nuclear receptor FXR inhibits Glucagon-Like Peptide-1 secretion in response to microbiota-derived Short-Chain Fatty Acids. Sci Rep. 2020;10(1):1–10. This important study draws a mechanistic link between intestinal SCFA and GLP-1 secretion by L-cells, underscoring the central role of the enteroendocrine system in regulating systemic GLP-1.
Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflamm. 2019;16(1):53.
Gwak MG, Chang SY. Gut-brain connection: microbiome, gut barrier, and environmental sensors. Immune Netw. 2021;21(3):e20.
Gutierrez-Aguilar R, Woods SC. Nutrition and L and K-enteroendocrine cells. Curr Opin Endocrinol Diabetes Obes. 2011;18(1):35–41.
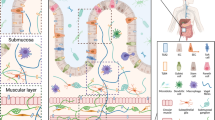
Worthington JJ, Reimann F, Gribble FM. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018;11(1):3–20.
Gribble FM, Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol. 2016;78:277–99.
Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15(4):226–37.
Spreckley E, Murphy KG. The L-cell in nutritional sensing and the regulation of appetite. Front Nutr. 2015;2:23.
Nadkarni P, Chepurny OG, Holz GG. Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci. 2014;121:23–65.
Aroda VR. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20 Suppl 1:22–33.
Gomez-Peralta F, Abreu C. Profile of semaglutide in the management of type 2 diabetes: design, development, and place in therapy. Drug Des Devel Ther. 2019;13:731–8.
Smilowitz NR, Donnino R, Schwartzbard A. Glucagon-like peptide-1 receptor agonists for diabetes mellitus: a role in cardiovascular disease. Circulation. 2014;129(22):2305–12.
Simon TG, Patorno E, Schneeweiss S. Glucose-like peptide-1 receptor agonists and hepatic decompensation events in patients with cirrhosis and diabetes. Clin Gastroenterol Hepatol. 2022;20(6):1382–93.
Fang Y, Jiang D, Wang Y, Wang Q, Lv D, Liu J, et al. Neuroprotection of rhGLP-1 in diabetic rats with cerebral ischemia/reperfusion injury via regulation of oxidative stress, EAAT2, and apoptosis. Drug Dev Res. 2018;79(6):249–59.
Hogg E, Athreya K, Basile C, Tan EE, Kaminski J, Tagliati M. High prevalence of undiagnosed insulin resistance in non-diabetic subjects with Parkinson’s disease. J Parkinsons Dis. 2018;8(2):259–65.
Nam GE, Kim SM, Han K, Kim NH, Chung HS, Kim JW, et al. Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med. 2018;15(8):e1002640.
Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH. A new treatment strategy for Parkinson’s disease through the gut–brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplant. 2017;26(9):1560–71.
Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: mechanisms of action. 2016;21(5):802–18.
Holscher C. Protective properties of GLP-1 and associated peptide hormones in neurodegenerative disorders. Br J Pharmacol. 2022;179(4):695–714.
Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, et al. Expression and distribution of glucagon-like peptide-1 receptor mrna, protein and binding in the male nonhuman primate (macaca mulatta) brain. Endocrinology. 2015;156(1):255–67.
Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol. 2009;202(3):431–9.
Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008;86(2):326–38.
Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med. 2018;24:931–38.
Ma D, Liu X, Liu J, Li M, Chen L, Gao M, et al. Long-term liraglutide ameliorates nigrostriatal impairment via regulating AMPK/PGC-1a signaling in diabetic mice. Brain Res. 2019;1714:126–32.
Badawi GA, Abd El Fattah MA, Zaki HF, El Sayed MI. Sitagliptin and liraglutide reversed nigrostriatal degeneration of rodent brain in rotenone-induced Parkinson’s disease. Inflammopharmacology. 2017;25(3):369–82.
Zhang L, Li L, Hölscher C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides. 2018;71:70–80.
Zhang L, Li L, Hölscher C. Semaglutide is neuroprotective and reduces α-synuclein levels in the chronic MPTP mouse model of Parkinson’s disease. J Parkinsons Dis. 2019;9(1):157–71.
Badawi GA, Abd El Fattah MA, Zaki HF, El Sayed MI. Sitagliptin and liraglutide modulate L-dopa effect and attenuate dyskinetic movements in rotenone-lesioned rats. Neurotox Res. 2019;35(3):635–53.
Fang X, Zhou X, Miao Y, Han Y, Wei J, Chen T. Therapeutic effect of GLP-1 engineered strain on mice model of Alzheimer’s disease and Parkinson’s disease. AMB Express. 2020;10:80.
Filchenko I, Simanenkova A, Chefu S, Kolpakova M, Vlasov T. Neuroprotective effect of glucagon-like peptide-1 receptor agonist is independent of glycaemia normalization in type two diabetic rats. Diab Vasc Dis Res. 2018;15(6):567–70.
Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson's disease: A randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664–75.
Athauda D, Maclagan K, Budnik N, Zampedri L, Hibbert S, Aviles-Olmos I, et al. Post hoc analysis of the Exenatide-PD trial—factors that predict response. Eur J Neurosci. 2019;49:410–21.
• Brauer R, Wei L, Ma T, Athauda D, Girges C, Vijiaratnam N, et al. Diabetes medications and risk of Parkinson’s disease: a cohort study of patients with diabetes. Brain. 2020;143(10):3067–76. This is the first study to provide sound epidemiological evidence for PD prevention among diabetics treated with GLP-1R agonists and DPP-4 inhibitors compared to other antidiabetic medications, corroborating earlier mechanistic studies demonstrating neuroprotective and anti-inflammatory effects of GLP-1R agonist treatment in PD.
Chen X, Huang Q, Feng J, Xiao Z, Zhang X, Zhao L. GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-κB signalling pathway. J Int Med Res. 2021;49(2):300060521992981.
Nuamnaichati N, Mangmool S, Chattipakorn N, Parichatikanond W. Stimulation of GLP-1 receptor inhibits methylglyoxal-induced mitochondrial dysfunctions in H9c2 cardiomyoblasts: potential role of Epac/PI3K/Akt pathway. Front Pharmacol. 2020;11:805.
YildirimSimsir I, Soyaltin UE, Cetinkalp S. Glucagon like peptide-1 (GLP-1) likes Alzheimer’s disease. Diabetes Metab Syndr. 2018;12(3):469–75.
Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54(1):10–8.
Greiner TU, Bäckhed F. Microbial regulation of GLP-1 and L-cell biology. Mol Metab. 2016;5(9):753–8.
Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–84.
Bishehsari F, Engen PA, Preite NZ, Tuncil YE, Naqib A, Shaikh M, et al. Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes. 2018;9:102.
De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–21.
Ohki J, Sakashita A, Aihara E, Inaba A, Uchiyama H, Matsumoto M, et al. Comparative analysis of enteroendocrine cells and their hormones between mouse intestinal organoids and native tissues. Biosci Biotechnol Biochem. 2020;84(5):936–42.
Sato T, Stange DE, Ferrante M, Vries RGJ, Van Es JH, Van Den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–72.
Goldspink DA, Lu VB, Miedzybrodzka EL, Smith CA, Foreman RE, Billing LJ, et al. Labeling and characterization of human GLP-1-secreting L-cells in primary ileal organoid culture. Cell Rep. 2020;31(13):107833.
Sato T, Vries RG, Snippert HJ, Van De Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5.
Petersen N, Reimann F, Bartfeld S, Farin HF, Ringnalda FC, Vries RGJ, et al. Generation of l cells in mouse and human small intestine organoids. Diabetes. 2014;63(2):410–20.
Altay G, Larrañaga E, Tosi S, Barriga FM, Batlle E, Fernández-Majada V, et al. Self-organized intestinal epithelial monolayers in crypt and villus-like domains show effective barrier function. Sci Rep. 2019;9(1):10140.
Co JY, Margalef-Català M, Li X, Mah AT, Kuo CJ, Monack DM, et al. Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep. 2019;26(9):2509-20.e4.
Co JY, Margalef-Català M, Monack DM, Amieva MR. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat Protoc. 2021;16(11):5171–92.
Xiang X, Wind K, Wiedemann T, Blume T, Shi Y, Briel N, et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci Transl Med. 2021;13(615):eabe5640.
Urizar NL, Liverman AB, Dodds DNT, Silva FV, Ordentlich P, Yan Y, et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296(5573):1703–6.
Acknowledgements
The authors would like to thank Mr. and Mrs. Larry Field, Mr. and Mrs. Glass, Mr. Keehn, Mr. B. Wu, Mr. Eric Larson, and the Alvin Baum Family fund for their philanthropic funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Movement Disorders
Rights and permissions
About this article
Cite this article
Manfready, R.A., Forsyth, C.B., Voigt, R.M. et al. Gut-Brain Communication in Parkinson’s Disease: Enteroendocrine Regulation by GLP-1. Curr Neurol Neurosci Rep 22, 335–342 (2022). https://doi.org/10.1007/s11910-022-01196-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01196-5