Abstract
Purpose of Review
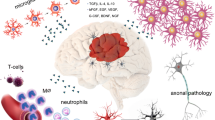
Traumatic brain injury (TBI) is amongst the leading causes of mortality and morbidity worldwide. However, several pharmacological strategies in the clinical setting remain unsuccessful. Mounting evidence implicates High Mobility Group Box protein 1 (HMGB1) as a unique alternative target following brain injury. Herein, we discuss current understanding of HMGB1 in TBI and obstacles to clinical translation.
Recent Findings
HMGB1 plays a pivotal role as a ‘master-switch’ of neuro-inflammation following injury and in the regulation of neurogenesis during normal development. Animal models point towards the involvement of HMGB1 signalling in prolonged activation of glial cells and widespread neuronal death. Early experimental studies demonstrate positive effects of HMGB1 antagonism on both immunohistochemical and neuro-behavioural parameters following injury. Raised serum/CSF HMGB1 in humans is associated with poor outcomes post-TBI.
Summary
HMGB1 is a promising therapeutic target post-TBI. However, further studies elucidating receptor, cell, isoform, and temporal effects are required prior to clinical translation.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morbidity and mortality weekly report. 2009;58(16):421-6.
Ghawami H, Sadeghi S, Raghibi M, Rahimi-Movaghar V. Executive functioning of complicated-mild to moderate traumatic brain injury patients with frontal contusions. Appl Neuropsychol Adult. 2016:1–9. https://doi.org/10.1080/23279095.2016.1157078.
Nygren DeBoussard C, Lannsjo M, Stenberg M, Stalnacke BM, Godbolt AK. Behavioural problems in the first year after severe traumatic brain injury: a prospective multicentre study. Clin Rehabil. 2017;31(4):555–66. https://doi.org/10.1177/0269215516652184.
Lewis FD, Horn GJ. Depression following traumatic brain injury: impact on post-hospital residential rehabilitation outcomes. Neuro Rehabilit. 2017. https://doi.org/10.3233/NRE-161427.
Chan RC, Hoosain R, Lee TM, Fan YW, Fong D. Are there sub-types of attentional deficits in patients with persisting post-concussive symptoms? A cluster analytical study. Brain Inj. 2003;17(2):131–48. https://doi.org/10.1080/0269905021000010168.
Simon DW, McGeachy MJ, Bayır H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nature Rev Neurol. 2017;13(9):572. https://doi.org/10.1038/nrneurol.2017.116.
Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95(6):1246–65. https://doi.org/10.1016/j.neuron.2017.07.010.
Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280(1):74–82. https://doi.org/10.1111/imr.12601.
Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270(43):25752–61. https://doi.org/10.1074/jbc.270.43.25752.
Ueda T, Yoshida M. HMGB proteins and transcriptional regulation. Biochimica et Biophysica Acta. 2010;1799(1-2):114–8. https://doi.org/10.1016/j.bbagrm.2009.11.005.
Fink MP. Bench-to-bedside review: high-mobility group box 1 and critical illness. Crit Care (London, England). 2007;11(5):229. https://doi.org/10.1186/cc6088.
Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, et al. High mobility group 1 B-box mediates activation of human endothelium. J Internal Med. 2003;254(4):375–85. https://doi.org/10.1046/j.1365-2796.2003.01204.x.
He M, Bianchi ME, Coleman TR, Tracey KJ, Al-Abed Y. Exploring the biological functional mechanism of the HMGB1/TLR4/MD-2 complex by surface plasmon resonance. Mol Med. 2018;24(1):21. https://doi.org/10.1186/s10020-018-0023-8.
•• Manivannan S, Marei O, Elalfy O, Zaben M. Neurogenesis after traumatic brain injury - The complex role of HMGB1 and neuroinflammation. Neuropharmacology. 2021;183:108400. https://doi.org/10.1016/j.neuropharm.2020.108400Narrative review summarising the dual role of HMGB1 in brain injury and neurogenesis. Effects of concentration, context, HMGB1 isoforms, and target receptors are discussed.
Wang KY, Yu GF, Zhang ZY, Huang Q, Dong XQ. Plasma high-mobility group box 1 levels and prediction of outcome in patients with traumatic brain injury. Clinica Chimica Acta; Int J Clin Chem. 2012;413(21-22):1737–41. https://doi.org/10.1016/j.cca.2012.07.002.
Au AK, Aneja RK, Bell MJ, Bayir H, Feldman K, Adelson PD, et al. Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. J Neurotrauma. 2012;29(11):2013–21. https://doi.org/10.1089/neu.2011.2171.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5. https://doi.org/10.1038/nature00858.
Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, et al. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62(1):26–38. https://doi.org/10.1002/glia.22581.
•• Manivannan S, Harari B, Muzaffar M, Elalfy O, Hettipathirannahelage S, James Z, et al. Glycyrrhizin Blocks the Detrimental Effects of HMGB1 on Cortical Neurogenesis After Traumatic Neuronal Injury. Brain Sci. 2020;10(10):760. https://doi.org/10.3390/brainsci10100760In vitro study demonstrating that HMGB1 release following traumatic injury reduces neuronal numbers/proportions in primary neural stem cell/progenitor cultures via RAGE-dependent effect. Indicates that HMGB1 release post-TBI may hinder cortical neurogenesis.
Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, et al. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem. 2007;103(2):590–603. https://doi.org/10.1111/j.1471-4159.2007.04788.x.
Braun M, Vaibhav K, Saad NM, Fatima S, Vender JR, Baban B, et al. White matter damage after traumatic brain injury: a role for damage associated molecular patterns. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2614–26. https://doi.org/10.1016/j.bbadis.2017.05.020.
Chen X, Wu S, Chen C, Xie B, Fang Z, Hu W, et al. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-kappaB pathway following experimental traumatic brain injury. J Neuroinflammation. 2017;14(1):143. https://doi.org/10.1186/s12974-017-0917-3.
Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22(20):5551–60. https://doi.org/10.1093/emboj/cdg516.
Gao T, Chen Z, Chen H, Yuan H, Wang Y, Peng X, et al. Inhibition of HMGB1 mediates neuroprotection of traumatic brain injury by modulating the microglia/macrophage polarization. Biochem Biophys Res Commun. 2018;497(1):430–6. https://doi.org/10.1016/j.bbrc.2018.02.102.
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization—new prospects for brain repair. Nature Rev Neurol. 2015;11(1):56–64. https://doi.org/10.1038/nrneurol.2014.207.
Wang L, Wu J, Guo X, Huang X, Huang Q. RAGE plays a role in LPS-induced NF-κB activation and endothelial hyperpermeability. Sensors (Basel, Switzerland). 2017;17(4):722. https://doi.org/10.3390/s17040722.
Okuma Y, Liu K, Wake H, Liu R, Nishimura Y, Hui Z, et al. Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1-RAGE interaction. Neuropharmacology. 2014;85:18–26. https://doi.org/10.1016/j.neuropharm.2014.05.007.
Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol. 2012;72(3):373–84. https://doi.org/10.1002/ana.23602.
Pang H, Huang T, Song J, Li D, Zhao Y, Ma X. Inhibiting HMGB1 with glycyrrhizic acid protects brain injury after DAI via its anti-inflammatory effect. Mediators Inflamm. 2016;2016:4569521. https://doi.org/10.1155/2016/4569521.
Nishibori M, Wang D, Ousaka D, Wake H. High mobility group box-1 and blood-brain barrier disruption. Cells. 2020;9(12):2650. doi:https://doi.org/10.3390/cells9122650.
Chou DK, Zhang J, Smith FI, McCaffery P, Jungalwala FB. Developmental expression of receptor for advanced glycation end products (RAGE), amphoterin and sulfoglucuronyl (HNK-1) carbohydrate in mouse cerebellum and their role in neurite outgrowth and cell migration. J Neurochem. 2004;90(6):1389–401. https://doi.org/10.1111/j.1471-4159.2004.02609.x.
Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275(51):40096–105. https://doi.org/10.1074/jbc.M006993200.
Xue X, Chen X, Fan W, Wang G, Zhang L, Chen Z, et al. High-mobility group box 1 facilitates migration of neural stem cells via receptor for advanced glycation end products signaling pathway. Scientific Reports. 2018;8(1):4513. https://doi.org/10.1038/s41598-018-22672-4.
Okuma Y, Wake H, Teshigawara K, Takahashi Y, Hishikawa T, Yasuhara T, et al. Anti-high mobility group box 1 antibody therapy may prevent cognitive dysfunction after traumatic brain injury. World Neurosurg. 2019;122:e864–e71. https://doi.org/10.1016/j.wneu.2018.10.164.
Su X, Wang H, Zhao J, Pan H, Mao L. Beneficial effects of ethyl pyruvate through inhibiting high-mobility group box 1 expression and TLR4/NF-kappaB pathway after traumatic brain injury in the rat. Mediators Inflammation. 2011;2011:807142. https://doi.org/10.1155/2011/807142.
Evran S, Calis F, Akkaya E, Baran O, Cevik S, Katar S, et al. The effect of high mobility group box-1 protein on cerebral edema, blood-brain barrier, oxidative stress and apoptosis in an experimental traumatic brain injury model. Brain Res Bull. 2020;154:68–80. https://doi.org/10.1016/j.brainresbull.2019.10.013.
Gu XJ, Xu J, Ma BY, Chen G, Gu PY, Wei D, et al. Effect of glycyrrhizin on traumatic brain injury in rats and its mechanism. Chin J Traumatol = Zhonghua chuang shang za zhi. 2014;17(1):1–7.
Aneja RK, Alcamo AM, Cummings J, Vagni V, Feldman K, Wang Q, et al. Lack of benefit on brain edema, blood-brain barrier permeability, or cognitive outcome in global inducible high mobility group box 1 knockout mice despite tissue sparing after experimental traumatic brain injury. J Neurotrauma. 2019;36(2):360–9. https://doi.org/10.1089/neu.2018.5664.
Armstrong RC, Mierzwa AJ, Sullivan GM, Sanchez MA. Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury. Neuropharmacology. 2016;110(Pt B):654–9. https://doi.org/10.1016/j.neuropharm.2015.04.029.
•• Ved R, Sharouf F, Harari B, Muzaffar M, Manivannan S, Ormonde C, et al. Disulfide HMGB1 acts via TLR2/4 receptors to reduce the numbers of oligodendrocyte progenitor cells after traumatic injury in vitro. Sci Rep. 2021;11(1):6181. https://doi.org/10.1038/s41598-021-84932-0Seminal in vitro study demonstrating a detrimental effect of HMGB1 on oligodendrocyte progenitor cells via TLR2/4 following traumatic injury. Indicates that extensive HMGB1 release post-injury may hinder recovery through impaired white matter repair.
Gao TL, Yuan XT, Yang D, Dai HL, Wang WJ, Peng X, et al. Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. J Trauma Acute Care Surg. 2012;72(3):643–9. https://doi.org/10.1097/TA.0b013e31823c54a6.
Hung CH, Kee KM, Chen CH, Tseng PL, Tsai MC, Chen CH, et al. A randomized controlled trial of glycyrrhizin plus tenofovir vs. tenofovir in chronic hepatitis B with severe acute exacerbation. Clin Transl Gastroenterol. 2017;8(6):e104. https://doi.org/10.1038/ctg.2017.29.
Xu W, Li Y, Ju M, Lai W, Lu X, Shi H, et al. A multicenter, randomized, double-blind, placebo-controlled study of compound glycyrrhizin capsules combined with a topical corticosteroid in adults with chronic eczema. Evid Based Complement Alternat Med. 2020;2020:6127327. https://doi.org/10.1155/2020/6127327.
Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regulatory Toxicol Pharmacol: RTP. 2006;46(3):167–92. https://doi.org/10.1016/j.yrtph.2006.06.002.
Lin C-C, Weng M-T, Chung C-S, Liang C-C. The dramatic effect of intravenous glycyrrhizin on acute-on-chronic hepatic failure in chronic hepatitis B patients without liver cirrhosis. Advances Digestive Med. 2019;6(4):153–7. https://doi.org/10.1002/aid2.13131.
Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer’s ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Critic Care Med. 2001;29(8):1513–8. https://doi.org/10.1097/00003246-200108000-00003.
Shin JH, Kim ID, Kim SW, Lee HK, Jin Y, Park JH, et al. Ethyl pyruvate inhibits HMGB1 phosphorylation and release by chelating calcium. Mol Med. 2015;20(1):649–57. https://doi.org/10.2119/molmed.2014.00039.
Shi H, Wang HL, Pu HJ, Shi YJ, Zhang J, Zhang WT, et al. Ethyl pyruvate protects against blood-brain barrier damage and improves long-term neurological outcomes in a rat model of traumatic brain injury. CNS Neurosci Therapeutics. 2015;21(4):374–84. https://doi.org/10.1111/cns.12366.
Fink MP. Ethyl pyruvate: a novel anti-inflammatory agent. J Internal Med. 2007;261(4):349–62. https://doi.org/10.1111/j.1365-2796.2007.01789.x.
Yang L, Wang F, Yang L, Yuan Y, Chen Y, Zhang G, et al. HMGB1 a-box reverses brain edema and deterioration of neurological function in a traumatic brain injury mouse model. Cell Physiol Biochem. 2018;46(6):2532–42. https://doi.org/10.1159/000489659.
Walker D, Lue LF, Paul G, Patel A, Sabbagh MN. Receptor for advanced glycation endproduct modulators: a new therapeutic target in Alzheimer’s disease. Expert Opin Investig Drugs. 2015;24(3):393–9. https://doi.org/10.1517/13543784.2015.1001490.
Availability of Data and Material
N/A
Code Availability
N/A
Funding
Dr. Malik Zaben is a member of the BRAIN Unit, which is funded by the Welsh Government through Health and Care Research Wales.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This manuscript has been written in compliance with the ethical standards of Current Neurology and Neuroscience Reports.
Conflict of Interest
None
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurotrauma
Rights and permissions
About this article
Cite this article
Manivannan, S., Wales, E. & Zaben, M. The Role of HMGB1 in Traumatic Brain Injury—Bridging the Gap Between the Laboratory and Clinical Studies. Curr Neurol Neurosci Rep 21, 75 (2021). https://doi.org/10.1007/s11910-021-01158-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11910-021-01158-3