Abstract
Purpose of Review
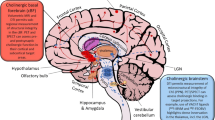
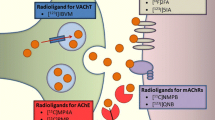
Brain cholinergic denervation is a major feature of Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB). We reviewed the topography assessed by a cholinergic molecular imaging study in these two major types of dementia. A small meta-analysis directly comparing vesicular acetylcholine transporter (VAChT) PET scans of AD vs. DLB patients is presented.
Recent Findings
VAChT PET studies showed evidence of extensive cortical cholinergic denervation in both forms of dementia, while multiple subcortical structures were also in DLB. Novel analysis revealed evidence of metathalamic denervation in AD, and epithalamus, premotor/sensorimotor cortical, and striatal losses in DLB.
Summary
Topographically distinct cortical and subcortical cholinergic lesions can distinguish AD and DLB, and new structures have been highlighted here. Differential vulnerability of specific cholinergic projections is likely associated with specific clinical features of these disorders. Improved understanding of the mechanisms and roles of cholinergic neurotransmission in regions with cholinergic deficits may lead to symptomatic therapies.



Similar content being viewed by others
Abbreviations
- AChE:
-
Acetylcholinesterase
- DLB:
-
Dementia with Lewy bodies
- FEOBV:
-
Fluoroethoxybenzovesamicol
- nbM:
-
Nucleus basalis of Meynert
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease dementia
- PET:
-
Positron emission tomography
- VAChT:
-
Vesicular acetylcholine transporter
- BF:
-
Basal forebrain
- PPN-LDT:
-
Pedunculopontine-laterodorsal tegmental complex
- MVN:
-
Medial vestibular nuclei
- SChIs:
-
Striatal cholinergic interneurons
- IBVM:
-
Iodobenzovesamicol
- VOI:
-
Volume of interest
- FDR:
-
False discovery rate
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bohnen NI, Grothe MJ, Ray NJ, Muller MLTM, Teipel SJ. Recent advances in cholinergic imaging and cognitive decline-revisiting the cholinergic hypothesis of dementia. Curr Geriatr Rep. 2018;7(1):1–11. https://doi.org/10.1007/s13670-018-0234-4.
Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur J Neurosci. 2014;39(11):1912–20. https://doi.org/10.1111/ejn.12515.
Gombkoto P, Gielow M, Varsanyi P, Chavez C, Zaborszky L. Contribution of the basal forebrain to corticocortical network interactions. Brain Struct Funct. 2021. https://doi.org/10.1007/s00429-021-02290-z.
Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, et al. In vivo imaging of human cholinergic nerve terminals with (-)-5-18F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med Off Publ Soc Nucl Med. 2014;55(3):396–404. https://doi.org/10.2967/jnumed.113.124792.
Albin RL, Bohnen NI, Muller M, Dauer WT, Sarter M, Frey KA, et al. Regional vesicular acetylcholine transporter distribution in human brain: a [(18) F]fluoroethoxybenzovesamicol positron emission tomography study. J Compar Neurol. 2018. https://doi.org/10.1002/cne.24541.
Mesulam MM. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J Comp Neurol. 2013;521(18):4124–44. https://doi.org/10.1002/cne.23415.
Arciniegas DB. Cholinergic dysfunction and cognitive impairment after traumatic brain injury Part 1: the structure and function of cerebral cholinergic systems. J Head Trauma Rehab. 2011;26(1):98–101. https://doi.org/10.1097/HTR.0b013e31820516cb.
Zhang C, Zhou P, Yuan T. The cholinergic system in the cerebellum: from structure to function. Rev Neurosci. 2016;27(8):769–76. https://doi.org/10.1515/revneuro-2016-0008.
Abudukeyoumu N, Hernandez-Flores T, Garcia-Munoz M, Arbuthnott GW. Cholinergic modulation of striatal microcircuits. Eur J Neurosci. 2019;49(5):604–22. https://doi.org/10.1111/ejn.13949.
Aghourian M, Legault-Denis C, Soucy JP, Rosa-Neto P, Gauthier S, Kostikov A, et al. Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [(18)F]-FEOBV. Mol Psychiatry. 2017;22(11):1531–8. https://doi.org/10.1038/mp.2017.183. This study reports the first detailed FEOBV PET analysis in patients with AD and reported widespread cortical reductions in VAChT binding.
Liu AK, Chang RC, Pearce RK, Gentleman SM. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015;129(4):527–40. https://doi.org/10.1007/s00401-015-1392-5.
Schmitz TW, Mur M, Aghourian M, Bedard MA, Spreng RN. Alzheimer’s Disease Neuroimaging I Longitudinal Alzheimer’s degeneration reflects the spatial topography of cholinergic basal forebrain projections. Cell reports. 2018;24(1):38–46. https://doi.org/10.1016/j.celrep.2018.06.001.
Lehericy S, Hirsch EC, Cervera-Pierot P, Hersh LB, Bakchine S, Piette F, et al. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J Comp Neurol. 1993;330(1):15–31. https://doi.org/10.1002/cne.903300103.
Mazere J, Lamare F, Allard M, Fernandez P, Mayo W. 123I-Iodobenzovesamicol SPECT imaging of cholinergic systems in dementia with Lewy bodies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017;58(1):123–8. https://doi.org/10.2967/jnumed.116.176180. First VAChT SPECT study in DLB.
Nejad-Davarani S, Koeppe RA, Albin RL, Frey KA, Muller M, Bohnen NI. Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [(18)F]-FEOBV. Mol Psychiatry. 2019;24(3):322–7. https://doi.org/10.1038/s41380-018-0130-5. This study reported VAChT binding losses in patients with DLB in the whole neocortex, hippocampus, amygdala, thalamus, basal ganglia, vermis, and dorsal pontomesenchephalic region.
Kanel P, Müller MLTM, van der Zee S, Sanchez-Catasus CA, Koeppe RA, Frey KA, et al. Topography of cholinergic changes in dementia with Lewy bodies and key neural network hubs. J Neuropsychiatry Clin Neurosci. 2020;32(4):370–5. https://doi.org/10.1176/appi.neuropsych.19070165. The authors used a different imaging analysis method (whole brain voxel-based analyses) and found a distinct topographic pattern of VAChT binding losses in patients with DLB. Regionally more prominent losses were seen in the bilateral opercula, anterior-to-mid cingulate cortices, bilateral insula, bilateral geniculate nuclei, pulvinar, right proximal optic radiation, bilateral anterior and superior thalami, and posterior hippocampal fimbria and fornix.
Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, et al. A new approach to spatial covariance modeling of functional brain imaging data: ordinal trend analysis. Neural Comput. 2005;17(7):1602–45. https://doi.org/10.1162/0899766053723023.
Schumacher J, Gunter JL, Przybelski SA, Jones DT, Graff-Radford J, Savica R, et al. Dementia with Lewy bodies: association of Alzheimer pathology with functional connectivity networks. Brain. 2021. https://doi.org/10.1093/brain/awab218.
Halassa MM, Kastner S. Thalamic functions in distributed cognitive control. Nat Neurosci. 2017;20(12):1669–79. https://doi.org/10.1038/s41593-017-0020-1.
Mihai PG, Moerel M, de Martino F, Trampel R, Kiebel S, von Kriegstein K. Modulation of tonotopic ventral medial geniculate body is behaviorally relevant for speech recognition. Elife. 2019;8. https://doi.org/10.7554/eLife.44837.
Phansuwan-Pujito P, Møller M, Govitrapong P. Cholinergic innervation and function in the mammalian pineal gland. Microsc Res Tech. 1999;46(4–5):281–95. https://doi.org/10.1002/(sici)1097-0029(19990815/01)46:4/5%3c281::Aid-jemt5%3e3.0.Co;2-n.
Heckers S, Geula C, Mesulam M. Cholinergic innervation of the human thalamus: dual origin and differential nuclear distribution. J Comp Neurol. 1992;325:68–82.
Lee SH, Cho H, Choi JY, Lee JH, Ryu YH, Lee MS, et al. Distinct patterns of amyloid-dependent tau accumulation in Lewy body diseases. Movement Disorders Off J Movement Disorder Soc. 2018;33(2):262–72. https://doi.org/10.1002/mds.27252.
Seidel K, Bouzrou M, Heidemann N, Krüger R, Schöls L, den Dunnen WFA, et al. Involvement of the cerebellum in Parkinson disease and dementia with Lewy bodies. Ann Neurol. 2017;81(6):898–903. https://doi.org/10.1002/ana.24937.
Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60(12):1745–8. https://doi.org/10.1001/archneur.60.12.1745.
Kuhl DE, Minoshima S, Fessler JA, Frey KA, Foster NL, Ficaro EP, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann Neurol. 1996;40(3):399–410. https://doi.org/10.1002/ana.410400309.
Hirano S, Shinotoh H, Shimada H, Ota T, Sato K, Tanaka N, et al. Voxel-based acetylcholinesterase PET study in early and late onset Alzheimer’s disease. J Alzheimers Dis. 2018;62(4):1539–48. https://doi.org/10.3233/jad-170749.
Hanna Al-Shaikh FS, Duara R, Crook JE, Lesser ER, Schaeverbeke J, Hinkle KM, et al. Selective vulnerability of the nucleus basalis of Meynert among neuropathologic subtypes of Alzheimer disease. JAMA Neurol. 2020;77(2):225–33. https://doi.org/10.1001/jamaneurol.2019.3606.
Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65(11):1716–22. https://doi.org/10.1212/01.wnl.0000191154.78131.f6.
van der Zee S, Vállez García D, Elsinga PH, Willemsen ATM, Boersma HH, Gerritsen MJJ, et al. [18F]Fluoroethoxybenzovesamicol in Parkinson’s disease patients: quantification of a novel cholinergic positron emission tomography tracer. Movement Disorders Off J Movement Disorder Soc. 2019;34(6):924–6. https://doi.org/10.1002/mds.27698.
van der Zee S, Muller M, Kanel P, van Laar T, Bohnen NI. Cholinergic denervation patterns across cognitive domains in Parkinson’s disease. Movement Disorders Off J Movement Disorder Soc. 2021;36(3):642–50. https://doi.org/10.1002/mds.28360.
Fu H, Hardy J, Duff KE. Selective vulnerability in neurodegenerative diseases. Nat Neurosci. 2018;21(10):1350–8. https://doi.org/10.1038/s41593-018-0221-2.
Acknowledgements
The authors acknowledge support from the National Institute of Health (NIH), Department of Veterans Affairs, the Parkinson’s Foundation, Michael J. Fox Foundation, the Canadian Institutes of Health Research (CIHR), and the Fond de Recherche du Québec en Santé (FRQS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest relevant to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dementia
Rights and permissions
About this article
Cite this article
Kanel, P., Bedard, MA., Aghourian, M. et al. Molecular Imaging of the Cholinergic System in Alzheimer and Lewy Body Dementias: Expanding Views. Curr Neurol Neurosci Rep 21, 52 (2021). https://doi.org/10.1007/s11910-021-01140-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11910-021-01140-z