Abstract
Purpose of Review
Since the advent of next-generation sequencing, the number of genes associated with dystonia has been growing exponentially. We provide here a comprehensive review of the latest genetic discoveries in the field of dystonia and discuss how the growing knowledge of biology underlying monogenic dystonias may influence and challenge current classification systems.
Recent Findings
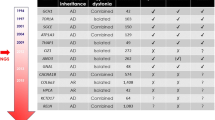
Pathogenic variants in genes without previously confirmed roles in human disease have been identified in subjects affected by isolated or combined dystonia (KMT2B, VPS16, HPCA, KCTD17, DNAJC12, SLC18A2) and complex dystonia (SQSTM1, IRF2BPL, YY1, VPS41). Importantly, the classical distinction between isolated and combined dystonias has become harder to sustain since many genes have been shown to determine multiple dystonic presentations (e.g., ANO3, GNAL, ADCY5, and ATP1A3). In addition, a growing number of genes initially linked to other neurological phenotypes, such as developmental delay, epilepsy, or ataxia, are now recognized to cause prominent dystonia, occasionally in an isolated fashion (e.g., GNAO1, GNB1, SCN8A, RHOBTB2, and COQ8A). Finally, emerging analyses suggest biological convergence of genes linked to different dystonic phenotypes.
Summary
While our knowledge on the genetic basis of monogenic dystonias has tremendously grown, their clinical boundaries are becoming increasingly blurry. The current phenotype-based classification may not reflect the molecular structure of the disease, urging the need for new systems based on shared biological pathways among dystonia-linked genes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28(7):863–73. https://doi.org/10.1002/mds.25475.
Marras C, Lang A, van de Warrenburg BP, Sue CM, Tabrizi SJ, Bertram L, et al. Nomenclature of genetic movement disorders: recommendations of the International Parkinson and Movement Disorder Society Task Force. Mov Disord. 2016;31(4):436–57. https://doi.org/10.1002/mds.26527.
Lohmann K, Klein C. Update on the genetics of dystonia. Curr Neurol Neurosci Rep. 2017;17(3):26. https://doi.org/10.1007/s11910-017-0735-0.
Barbagiovanni G, Germain PL, Zech M, Atashpaz S, Lo Riso P, D'Antonio-Chronowska A, et al. KMT2B is selectively required for neuronal transdifferentiation, and its loss exposes dystonia candidate genes. Cell Rep. 2018;25(4):988–1001. https://doi.org/10.1016/j.celrep.2018.09.067.
Meyer E, Carss KJ, Rankin J, Nichols JM, Grozeva D, Joseph AP, et al. Mutations in the histone methyltransferase gene KMT2B cause complex early-onset dystonia. Nat Genet. 2017;49(2):223–37. https://doi.org/10.1038/ng.3740.
Zech M, Boesch S, Maier EM, Borggraefe I, Vill K, Laccone F, et al. Haploinsufficiency of KMT2B, encoding the lysine-specific histone methyltransferase 2B, results in early-onset generalized dystonia. Am J Hum Genet. 2016;99(6):1377–87. https://doi.org/10.1016/j.ajhg.2016.10.010.
Li XY, Dai LF, Wan XH, Guo Y, Dai Y, Li SL, et al. Clinical phenotypes, genotypes and treatment in Chinese dystonia patients with KMT2B variants. Parkinsonism Relat Disord. 2020;77:76–82. https://doi.org/10.1016/j.parkreldis.2020.06.002.
Ng A, Galosi S, Salz L, Wong T, Schwager C, Amudhavalli S, et al. Failure to thrive - an overlooked manifestation of KMT2B-related dystonia: a case presentation. BMC Neurol. 2020;20(1):246. https://doi.org/10.1186/s12883-020-01798-x.
Dai L, Ding C, Fang F. An inherited KMT2B duplication variant in a Chinese family with dystonia and/or development delay. Parkinsonism Relat Disord. 2019;63:227–8. https://doi.org/10.1016/j.parkreldis.2018.08.021.
Dafsari HS, Sprute R, Wunderlich G, Daimaguler HS, Karaca E, Contreras A, et al. Novel mutations in KMT2B offer pathophysiological insights into childhood-onset progressive dystonia. J Hum Genet. 2019;64(8):803–13. https://doi.org/10.1038/s10038-019-0625-1.
Zhou XY, Wu JJ, Sun YM. An atypical case of early-onset dystonia with a novel missense variant in KMT2B. Parkinsonism Relat Disord. 2019;63:224–6. https://doi.org/10.1016/j.parkreldis.2018.09.020.
Bras A, Ribeiro JA, Sobral F, Moreira F, Morgadinho A, Januario C. Early-onset oromandibular-laryngeal dystonia and Charlot gait: new phenotype of DYT-KMT2B. Neurology. 2019;92(19):919. https://doi.org/10.1212/WNL.0000000000007469.
Carecchio M, Invernizzi F, Gonzalez-Latapi P, Panteghini C, Zorzi G, Romito L, et al. Frequency and phenotypic spectrum of KMT2B dystonia in childhood: a single-center cohort study. Mov Disord. 2019;34(10):1516–27. https://doi.org/10.1002/mds.27771The authors of this paper report 14 patients with KMT2B-related disease, highlighting its wide phenotypical heterogeneity that includes short stature and intellectual disability without evidence of dystonia in some cases. Addtionally, they describe a patient carrying a mutation in GNAO1 affected by myoclonus-dystonia in absence of epilepsy or other neurological manifestations.
Ma J, Wang L, Yang Y, Li S, Wan X. Identification of novel KMT2B variants in Chinese dystonia patients via whole-exome sequencing. Front Neurol. 2019;10:729. https://doi.org/10.3389/fneur.2019.00729.
Zech M, Lam DD, Winkelmann J. Update on KMT2B-related dystonia. Curr Neurol Neurosci Rep. 2019;19(11):92. https://doi.org/10.1007/s11910-019-1007-y.
Faundes V, Newman WG, Bernardini L, Canham N, Clayton-Smith J, Dallapiccola B, et al. Histone lysine methylases and demethylases in the landscape of human developmental disorders. Am J Hum Genet. 2018;102(1):175–87. https://doi.org/10.1016/j.ajhg.2017.11.013.
Kawarai T, Miyamoto R, Nakagawa E, Koichihara R, Sakamoto T, Mure H, et al. Phenotype variability and allelic heterogeneity in KMT2B-associated disease. Parkinsonism Relat Disord. 2018;52:55–61. https://doi.org/10.1016/j.parkreldis.2018.03.022.
Cao Z, Yao H, Bao X, Wen Y, Liu B, Wang S, et al. DYT28 responsive to pallidal deep brain stimulation. Mov Disord Clin Pract. 2020;7(1):97–9. https://doi.org/10.1002/mdc3.12862.
Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, et al. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell. 2014;25(8):1327–37. https://doi.org/10.1091/mbc.E13-08-0447.
Steel D, Zech M, Zhao C, Barwick KE, Burke D, Demailly D, et al. Loss-of-function variants in HOPS complex genes VPS16 and VPS41 cause early-onset dystonia associated with lysosomal abnormalities. Ann Neurol. 2020. https://doi.org/10.1002/ana.25879Steel et al. described patients with dystonia harboring loss-of-function mutations in VPS16 and VPS41, two genes involved in autophagosome-lysosome fusion. This study highlights the role of HOPS complex dysfunction in isolated dystonia pathogenesis.
Cai X, Chen X, Wu S, Liu W, Zhang X, Zhang D, et al. Homozygous mutation of VPS16 gene is responsible for an autosomal recessive adolescent-onset primary dystonia. Sci Rep. 2016;6:25834. https://doi.org/10.1038/srep25834.
van der Welle REN, Jobling R, Burns C, Sanza P, ten Brink C, Fasano A, et al. VPS41 recessive mutation causes ataxia and dystonia with retinal dystrophy and mental retardation by inhibiting HOPS function and mTORC1 signaling. bioRxiv. 2019:2019.12.18.867333. https://doi.org/10.1101/2019.12.18.867333.
Helassa N, Antonyuk SV, Lian LY, Haynes LP, Burgoyne RD. Biophysical and functional characterization of hippocalcin mutants responsible for human dystonia. Hum Mol Genet. 2017;26(13):2426–35. https://doi.org/10.1093/hmg/ddx133.
Kim KS, Kobayashi M, Takamatsu K, Tzingounis AV. Hippocalcin and KCNQ channels contribute to the kinetics of the slow after hyperpolarization. Biophys J. 2012;103(12):2446–54. https://doi.org/10.1016/j.bpj.2012.11.002.
Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow after hyperpolarization in hippocampal pyramidal cells. Neuron. 2007;53(4):487–93. https://doi.org/10.1016/j.neuron.2007.01.011.
Charlesworth G, Angelova PR, Bartolome-Robledo F, Ryten M, Trabzuni D, Stamelou M, et al. Mutations in HPCA cause autosomal-recessive primary isolated dystonia. Am J Hum Genet. 2015;96(4):657–65. https://doi.org/10.1016/j.ajhg.2015.02.007.
Dobricic V, Kresojevic N, Marjanovic A, Tomic A, Svetel M, Novakovic I, et al. HPCA-related dystonia: too rare to be found? Mov Disord. 2016;31(7):1071. https://doi.org/10.1002/mds.26634.
Carecchio M, Reale C, Invernizzi F, Monti V, Petrucci S, Ginevrino M, et al. DYT2 screening in early-onset isolated dystonia. Eur J Paediatr Neurol. 2017;21(2):269–71. https://doi.org/10.1016/j.ejpn.2016.10.001.
Atasu B, Hanagasi H, Bilgic B, Pak M, Erginel-Unaltuna N, Hauser AK, et al. HPCA confirmed as a genetic cause of DYT2-like dystonia phenotype. Mov Disord. 2018;33(8):1354–8. https://doi.org/10.1002/mds.27442This is the second paper to report cases affected by dystonia harboring homozygous truncating mutations in HPCA, confirming the role of loss-of-function of this gene in autosomal recessive dystonia.
Osypenko DS, Dovgan AV, Kononenko NI, Dromaretsky AV, Matvieienko M, Rybachuk OA, et al. Perturbed Ca(2+)-dependent signaling of DYT2 hippocalcin mutant as mechanism of autosomal recessive dystonia. Neurobiol Dis. 2019;132:104529. https://doi.org/10.1016/j.nbd.2019.104529.
Li Q, Kellner DA, Hatch HAM, Yumita T, Sanchez S, Machold RP, et al. Conserved properties of drosophila insomniac link sleep regulation and synaptic function. PLoS Genet. 2017;13(5):e1006815. https://doi.org/10.1371/journal.pgen.1006815.
Mencacci NE, Rubio-Agusti I, Zdebik A, Asmus F, Ludtmann MH, Ryten M, et al. A missense mutation in KCTD17 causes autosomal dominant myoclonus-dystonia. Am J Hum Genet. 2015;96(6):938–47. https://doi.org/10.1016/j.ajhg.2015.04.008.
Graziola F, Stregapede F, Travaglini L, Garone G, Verardo M, Bosco L, et al. A novel KCTD17 mutation is associated with childhood early-onset hyperkinetic movement disorder. Parkinsonism Relat Disord. 2019;61:4–6. https://doi.org/10.1016/j.parkreldis.2018.12.001Describe the identification splice-site mutations in KCTD17 in patients with myoclonus-dystonia, thus confirming the pathogenic role of this gene.
Marce-Grau A, Correa M, Vanegas MI, Munoz-Ruiz T, Ferrer-Aparicio S, Baide H, et al. Childhood onset progressive myoclonic dystonia due to a de novo KCTD17 splicing mutation. Parkinsonism Relat Disord. 2019;61:7–9. https://doi.org/10.1016/j.parkreldis.2019.01.004Describe the identification splice-site mutations in KCTD17 in patients with myoclonus-dystonia, thus confirming the pathogenic role of this gene.
Todisco M, Gana S, Cosentino G, Errichiello E, Arceri S, Avenali M, et al. KCTD17-related myoclonus-dystonia syndrome: clinical and electrophysiological findings of a patient with atypical late onset. Parkinsonism Relat Disord. 2020;78:129–33. https://doi.org/10.1016/j.parkreldis.2020.07.026.
Wijemanne S, Jankovic J. Dopa-responsive dystonia--clinical and genetic heterogeneity. Nat Rev Neurol. 2015;11(7):414–24. https://doi.org/10.1038/nrneurol.2015.86.
Anikster Y, Haack TB, Vilboux T, Pode-Shakked B, Thony B, Shen N, et al. Biallelic mutations in DNAJC12 cause hyperphenylalaninemia, dystonia, and intellectual disability. Am J Hum Genet. 2017;100(2):257–66. https://doi.org/10.1016/j.ajhg.2017.01.002This paper reported for the first time the association between mutations in DNAJC12 and dopa-resposnive dystonia, adding DNAJC12-related disease in the differential diagnosis of congenital hyperphenylalaninemia.
van Spronsen FJ, Himmelreich N, Rufenacht V, Shen N, Vliet DV, Al-Owain M, et al. Heterogeneous clinical spectrum of DNAJC12-deficient hyperphenylalaninemia: from attention deficit to severe dystonia and intellectual disability. J Med Genet. 2017;55:249–53. https://doi.org/10.1136/jmedgenet-2017-104875.
Veenma D, Cordeiro D, Sondheimer N, Mercimek-Andrews S. DNAJC12-associated developmental delay, movement disorder, and mild hyperphenylalaninemia identified by whole-exome sequencing re-analysis. Eur J Hum Genet. 2018;26(12):1867–70. https://doi.org/10.1038/s41431-018-0237-9.
Straniero L, Guella I, Cilia R, Parkkinen L, Rimoldi V, Young A, et al. DNAJC12 and dopa-responsive nonprogressive parkinsonism. Ann Neurol. 2017;82(4):640–6. https://doi.org/10.1002/ana.25048.
Rilstone JJ, Alkhater RA, Minassian BA. Brain dopamine-serotonin vesicular transport disease and its treatment. N Engl J Med. 2013;368(6):543–50. https://doi.org/10.1056/NEJMoa1207281.
Jacobsen JC, Wilson C, Cunningham V, Glamuzina E, Prosser DO, Love DR, et al. Brain dopamine-serotonin vesicular transport disease presenting as a severe infantile hypotonic parkinsonian disorder. J Inherit Metab Dis. 2016;39(2):305–8. https://doi.org/10.1007/s10545-015-9897-6.
Padmakumar M, Jaeken J, Ramaekers V, Lagae L, Greene D, Thys C, et al. A novel missense variant in SLC18A2 causes recessive brain monoamine vesicular transport disease and absent serotonin in platelets. JIMD Rep. 2019;47(1):9–16. https://doi.org/10.1002/jmd2.12030.
Rath M, Korenke GC, Najm J, Hoffmann GF, Hagendorff A, Strom TM, et al. Exome sequencing results in identification and treatment of brain dopamine-serotonin vesicular transport disease. J Neurol Sci. 2017;379:296–7. https://doi.org/10.1016/j.jns.2017.06.034.
van Egmond ME, Kuiper A, Eggink H, Sinke RJ, Brouwer OF, Verschuuren-Bemelmans CC, et al. Dystonia in children and adolescents: a systematic review and a new diagnostic algorithm. J Neurol Neurosurg Psychiatry. 2015;86(7):774–81. https://doi.org/10.1136/jnnp-2014-309106.
Haack TB, Ignatius E, Calvo-Garrido J, Iuso A, Isohanni P, Maffezzini C, et al. Absence of the autophagy adaptor SQSTM1/p62 causes childhood-onset neurodegeneration with ataxia, dystonia, and gaze palsy. Am J Hum Genet. 2016;99(3):735–43. https://doi.org/10.1016/j.ajhg.2016.06.026This paper describes the association between mutations in SQSTM1 and complex dystonia and provides experimental evidence of autophagic dysfunction in patient’s fibroblasts.
Muto V, Flex E, Kupchinsky Z, Primiano G, Galehdari H, Dehghani M, et al. Biallelic SQSTM1 mutations in early-onset, variably progressive neurodegeneration. Neurology. 2018;91(4):e319–e30. https://doi.org/10.1212/WNL.0000000000005869.
Zúñiga-Ramírez C, de Oliveira LM, Kramis-Hollands M, Algarni M, Soto-Escageda A, Sáenz-Farret M, et al. Beyond dystonia and ataxia: expanding the phenotype of SQSTM1 mutations. Parkinsonism Relat Disord. 2019;62:192–5. https://doi.org/10.1016/j.parkreldis.2018.12.031.
Marcogliese PC, Shashi V, Spillmann RC, Stong N, Rosenfeld JA, Koenig MK, et al. IRF2BPL is associated with neurological phenotypes. Am J Hum Genet. 2018;103(2):245–60. https://doi.org/10.1016/j.ajhg.2018.07.006Marcogliese et al. identify for the first time IRF2BPL as the cause of a neurodevelopmental regression syndrome characterized by the presence of a prominent complex movement disorder often featuring dystonia.
Prilop L, Buchert R, Woerz S, Gerloff C, Haack TB, Zittel S. IRF2BPL mutation causes nigrostriatal degeneration presenting with dystonia, spasticity and keratoconus. Parkinsonism Relat Disord. 2020;79:141–3. https://doi.org/10.1016/j.parkreldis.2020.03.030.
Ganos C, Zittel S, Hidding U, Funke C, Biskup S, Bhatia KP. IRF2BPL mutations cause autosomal dominant dystonia with anarthria, slow saccades and seizures. Parkinsonism Relat Disord. 2019;68:57–9. https://doi.org/10.1016/j.parkreldis.2019.09.020.
Skorvanek M, Dusek P, Rydzanicz M, Walczak A, Kosinska J, Kostrzewa G, et al. Neurodevelopmental disorder associated with IRF2BPL gene mutation: expanding the phenotype? Parkinsonism Relat Disord. 2019;62:239–41. https://doi.org/10.1016/j.parkreldis.2019.01.017.
Ginevrino M, Battini R, Nuovo S, Simonati A, Micalizzi A, Contaldo I, et al. A novel IRF2BPL truncating variant is associated with endolysosomal storage. Mol Biol Rep. 2020;47(1):711–4. https://doi.org/10.1007/s11033-019-05109-7.
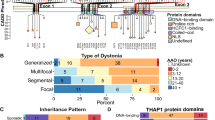
Yellajoshyula D, Liang CC, Pappas SS, Penati S, Yang A, Mecano R, et al. The DYT6 dystonia protein THAP1 regulates myelination within the oligodendrocyte lineage. Dev Cell. 2017;42(1):52–67 e4. https://doi.org/10.1016/j.devcel.2017.06.009.
Vissers LE, de Ligt J, Gilissen C, Janssen I, Steehouwer M, de Vries P, et al. A de novo paradigm for mental retardation. Nat Genet. 2010;42(12):1109–12. https://doi.org/10.1038/ng.712.
Gabriele M, Vulto-van Silfhout AT, Germain PL, Vitriolo A, Kumar R, Douglas E, et al. YY1 haploinsufficiency causes an intellectual disability syndrome featuring transcriptional and chromatin dysfunction. Am J Hum Genet. 2017;100(6):907–25. https://doi.org/10.1016/j.ajhg.2017.05.006.
Carminho-Rodrigues MT, Steel D, Sousa SB, Brandt G, Guipponi M, Laurent S, et al. Complex movement disorder in a patient with heterozygous YY1 mutation (Gabriele-de Vries syndrome). Am J Med Genet A. 2020;182:2129–32. https://doi.org/10.1002/ajmg.a.61731.
Xiao J, Uitti RJ, Zhao Y, Vemula SR, Perlmutter JS, Wszolek ZK, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71(4):458–69. https://doi.org/10.1002/ana.23547.
Dufke C, Hauser AK, Sturm M, Fluhr S, Wachter T, Leube B, et al. Mutations in CIZ1 are not a major cause for dystonia in Germany. Mov Disord. 2015;30(5):740–3. https://doi.org/10.1002/mds.26198.
Ma L, Chen R, Wang L, Yang Y, Wan X. No mutations in CIZ1 in twelve adult-onset primary cervical dystonia families. Mov Disord. 2013;28(13):1899–901. https://doi.org/10.1002/mds.25542.
Hammer M, Abravanel A, Peckham E, Mahloogi A, Majounie E, Hallett M, et al. Blepharospasm: a genetic screening study in 132 patients. Parkinsonism Relat Disord. 2019;64:315–8. https://doi.org/10.1016/j.parkreldis.2019.04.003.
Zech M, Lam DD, Francescatto L, Schormair B, Salminen AV, Jochim A, et al. Recessive mutations in the alpha3 (VI) collagen gene COL6A3 cause early-onset isolated dystonia. Am J Hum Genet. 2015;96(6):883–93. https://doi.org/10.1016/j.ajhg.2015.04.010.
Lohmann K, Schlicht F, Svetel M, Hinrichs F, Zittel S, Graf J, et al. The role of mutations in COL6A3 in isolated dystonia. J Neurol. 2016;263(4):730–4. https://doi.org/10.1007/s00415-016-8046-y.
Groen JL, Andrade A, Ritz K, Jalalzadeh H, Haagmans M, Bradley TE, et al. CACNA1B mutation is linked to unique myoclonus-dystonia syndrome. Hum Mol Genet. 2015;24(4):987–93. https://doi.org/10.1093/hmg/ddu513.
Mencacci NE, R'Bibo L, Bandres-Ciga S, Carecchio M, Zorzi G, Nardocci N, et al. The CACNA1B R1389H variant is not associated with myoclonus-dystonia in a large European multicentric cohort. Hum Mol Genet. 2015;24(18):5326–9. https://doi.org/10.1093/hmg/ddv255.
Gorman KM, Meyer E, Grozeva D, Spinelli E, McTague A, Sanchis-Juan A, et al. Bi-allelic loss-of-function CACNA1B mutations in progressive epilepsy-dyskinesia. Am J Hum Genet. 2019;104(5):948–56. https://doi.org/10.1016/j.ajhg.2019.03.005.
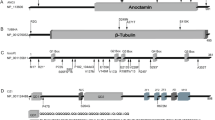
Kuo MC, Lin HI, Lin CH. Craniocervical dystonia with levodopa-responsive parkinsonism co-segregating with a pathogenic ANO3 mutation in a Taiwanese family. Parkinsonism Relat Disord. 2019;62:236–8. https://doi.org/10.1016/j.parkreldis.2019.01.020.
Yoo D, Kim HJ, Lee JS, Lee S, Kim SY, Choi M, et al. Early-onset generalized dystonia starting in the lower extremities in a patient with a novel ANO3 variant. Parkinsonism Relat Disord. 2018;50:124–5. https://doi.org/10.1016/j.parkreldis.2018.02.012.
Zech M, Boesch S, Jochim A, Weber S, Meindl T, Schormair B, et al. Clinical exome sequencing in early-onset generalized dystonia and large-scale resequencing follow-up. Mov Disord. 2017;32(4):549–59. https://doi.org/10.1002/mds.26808.
Geoghegan AR, Al Hussona M, Beauchamp NJ, Hutchinson M, Sean O'Riordan MB, Lynch T, et al. A novel GNAL mutation in familial dystonia presenting with childhood tremor and myoclonus. Mov Disord. 2019;34(6):923–4. https://doi.org/10.1002/mds.27694.
Chen YZ, Matsushita MM, Robertson P, Rieder M, Girirajan S, Antonacci F, et al. Autosomal dominant familial dyskinesia and facial myokymia: single exome sequencing identifies a mutation in adenylyl cyclase 5. Arch Neurol. 2012;69(5):630–5. https://doi.org/10.1001/archneurol.2012.54.
Chen DH, Meneret A, Friedman JR, Korvatska O, Gad A, Bonkowski ES, et al. ADCY5-related dyskinesia: broader spectrum and genotype-phenotype correlations. Neurology. 2015;85(23):2026–35. https://doi.org/10.1212/WNL.0000000000002058.
Douglas AG, Andreoletti G, Talbot K, Hammans SR, Singh J, Whitney A, et al. ADCY5-related dyskinesia presenting as familial myoclonus-dystonia. Neurogenetics. 2017;18(2):111–7. https://doi.org/10.1007/s10048-017-0510-z.
Carecchio M, Mencacci NE, Iodice A, Pons R, Panteghini C, Zorzi G, et al. ADCY5-related movement disorders: frequency, disease course and phenotypic variability in a cohort of paediatric patients. Parkinsonism Relat Disord. 2017;41:37–43. https://doi.org/10.1016/j.parkreldis.2017.05.004This manuscript hihglights that ADCY5 mutations are responsible for a variety of childhood-onset hyperkinetic movement disorders, including myoclonus-dystonia.
Friedman JR, Meneret A, Chen DH, Trouillard O, Vidailhet M, Raskind WH, et al. ADCY5 mutation carriers display pleiotropic paroxysmal day and nighttime dyskinesias. Mov Disord. 2016;31(1):147–8. https://doi.org/10.1002/mds.26494.
Carecchio M, Zorzi G, Ragona F, Zibordi F, Nardocci N. ATP1A3-related disorders: an update. Eur J Paediatr Neurol. 2018;22(2):257–63. https://doi.org/10.1016/j.ejpn.2017.12.009.
Balint B, Stephen CD, Udani V, Sankhla CS, Barad NH, Lang AE, et al. Paroxysmal asymmetric dystonic arm posturing-a less recognized but characteristic manifestation of ATP1A3-related disease. Mov Disord Clin Pract. 2019;6(4):312–5. https://doi.org/10.1002/mdc3.12747.
Jo S, Park KW, Choi N, Ryu HS, Kim K, Kim YJ, et al. Paroxysmal generalized dystonia with clinical fluctuation affected by the menstrual cycle. Parkinsonism Relat Disord. 2020;75:48–9. https://doi.org/10.1016/j.parkreldis.2020.05.009.
Zúñiga-Ramírez C, Kramis-Hollands M, Mercado-Pimentel R, González-Usigli HA, Sáenz-Farret M, Soto-Escageda A, et al. Generalized dystonia and paroxysmal dystonic attacks due to a novel ATP1A3 variant. Tremor and Other hyperkinetic movements (New York, NY). 2019;9. https://doi.org/10.7916/tohm.v0.723.
Sasaki M, Sumitomo N, Shimizu-Motohashi Y, Takeshita E, Kurosawa K, Kosaki K, et al. ATP1A3 variants and slowly progressive cerebellar ataxia without paroxysmal or episodic symptoms in children. Dev Med Child Neurol. 2020;63:111–5. https://doi.org/10.1111/dmcn.14666.
Schirinzi T, Graziola F, Cusmai R, Fusco L, Nicita F, Elia M, et al. ATP1A3-related epileptic encephalopathy responding to ketogenic diet. Brain and Development. 2018;40(5):433–8. https://doi.org/10.1016/j.braindev.2018.01.002.
Levy A, Lang AE. Ataxia-telangiectasia: a review of movement disorders, clinical features, and genotype correlations. Mov Disord. 2018;33(8):1238–47. https://doi.org/10.1002/mds.27319.
Mollet J, Delahodde A, Serre V, Chretien D, Schlemmer D, Lombes A, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82(3):623–30. https://doi.org/10.1016/j.ajhg.2007.12.022.
Galosi S, Barca E, Carrozzo R, Schirinzi T, Quinzii CM, Lieto M, et al. Dystonia-Ataxia with early handwriting deterioration in COQ8A mutation carriers: a case series and literature review. Parkinsonism Relat Disord. 2019;68:8–16. https://doi.org/10.1016/j.parkreldis.2019.09.015.
Traschütz A, Schirinzi T, Laugwitz L, Murray NH, Bingman CA, Reich S, et al. Clinico-genetic, imaging and molecular delineation of COQ8A-ataxia: a multicenter study of 59 patients. Ann Neurol. 2020;88:251–63. https://doi.org/10.1002/ana.25751.
Ebrahimi-Fakhari D, Saffari A, Westenberger A, Klein C. The evolving spectrum of PRRT2-associated paroxysmal diseases. Brain. 2015;138(Pt 12):3476–95. https://doi.org/10.1093/brain/awv317.
Gardiner AR, Jaffer F, Dale RC, Labrum R, Erro R, Meyer E, et al. The clinical and genetic heterogeneity of paroxysmal dyskinesias. Brain. 2015;138(Pt 12):3567–80. https://doi.org/10.1093/brain/awv310.
Tian WT, Huang XJ, Mao X, Liu Q, Liu XL, Zeng S, et al. Proline-rich transmembrane protein 2-negative paroxysmal kinesigenic dyskinesia: clinical and genetic analyses of 163 patients. Mov Disord. 2018;33(3):459–67. https://doi.org/10.1002/mds.27274The authors identify mutations in several genes responsible for PKD in cases without PRRT2 variants, including SCN8A and KCNA1.
Yin XM, Lin JH, Cao L, Zhang TM, Zeng S, Zhang KL, et al. Familial paroxysmal kinesigenic dyskinesia is associated with mutations in the KCNA1 gene. Hum Mol Genet. 2018;27(4):625–37. https://doi.org/10.1093/hmg/ddx430.
Fuerte-Hortigon A, Perez-Noguera R, Dotor Garcia-Soto J, Royo Boronat I, Alvarez de Andres S, Garcia-Moreno JM. Novel CACNA1A variant may cause cervical dystonia and cerebellar ataxia syndrome. J Neurol Sci. 2020;415:116909. https://doi.org/10.1016/j.jns.2020.116909.
Danielsson A, Anderlid BM, Stodberg T, Lagerstedt-Robinson K, Klackenberg Arrhenius E, Tedroff K. Benign paroxysmal torticollis of infancy does not lead to neurological sequelae. Dev Med Child Neurol. 2018;60(12):1251–5. https://doi.org/10.1111/dmcn.13939.
Tian J, Vemula SR, Xiao J, Valente EM, Defazio G, Petrucci S, et al. Whole-exome sequencing for variant discovery in blepharospasm. Mol Genet Genomic Med. 2018;6:601–26. https://doi.org/10.1002/mgg3.411.
Schirinzi T, Garone G, Travaglini L, Vasco G, Galosi S, Rios L, et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: results from an analytical review. Parkinsonism Relat Disord. 2019;61:19–25. https://doi.org/10.1016/j.parkreldis.2018.11.019This manuscript provides a comprehensive description of GNAO1-related movement disorders, underlining the considerable overlap between epileptic encephalopathies and dystonia ♦and movement disorders in general.
Danti FR, Galosi S, Romani M, Montomoli M, Carss KJ, Raymond FL, et al. GNAO1 encephalopathy: broadening the phenotype and evaluating treatment and outcome. Neurol Genet. 2017;3(2):e143. https://doi.org/10.1212/NXG.0000000000000143.
Hemati P, Revah-Politi A, Bassan H, Petrovski S, Bilancia CG, Ramsey K, et al. Refining the phenotype associated with GNB1 mutations: clinical data on 18 newly identified patients and review of the literature. Am J Med Genet A. 2018;176(11):2259–75. https://doi.org/10.1002/ajmg.a.40472.
Jones HF, Morales-Briceno H, Barwick K, Lewis J, Sanchis-Juan A, Raymond FL, et al. Myoclonus-dystonia caused by GNB1 mutation responsive to deep brain stimulation. Mov Disord. 2019;34(7):1079–80. https://doi.org/10.1002/mds.27708.
Steinrucke S, Lohmann K, Domingo A, Rolfs A, Baumer T, Spiegler J, et al. Novel GNB1 missense mutation in a patient with generalized dystonia, hypotonia, and intellectual disability. Neurol Genet. 2016;2(5):e106. https://doi.org/10.1212/NXG.0000000000000106.
Gardella E, Becker F, Møller RS, Schubert J, Lemke JR, Larsen LH, et al. Benign infantile seizures and paroxysmal dyskinesia caused by an SCN8A mutation. Ann Neurol. 2016;79(3):428–36. https://doi.org/10.1002/ana.24580.
Wagnon JL, Mencacci NE, Barker BS, Wengert ER, Bhatia KP, Balint B, et al. Partial loss-of-function of sodium channel SCN8A in familial isolated myoclonus. Hum Mutat. 2018;39(7):965–9. https://doi.org/10.1002/humu.23547.
Straub J, Konrad EDH, Gruner J, Toutain A, Bok LA, Cho MT, et al. Missense variants in RHOBTB2 cause a developmental and epileptic encephalopathy in humans, and altered levels cause neurological defects in drosophila. Am J Hum Genet. 2018;102(1):44–57. https://doi.org/10.1016/j.ajhg.2017.11.008.
Necpal J, Zech M, Valachova A, Sedlacek Z, Bendova S, Hancarova M, et al. Severe paroxysmal dyskinesias without epilepsy in a RHOBTB2 mutation carrier. Parkinsonism Relat Disord. 2020;77:87–8. https://doi.org/10.1016/j.parkreldis.2020.06.028.
Rittiner JE, Caffall ZF, Hernández-Martinez R, Sanderson SM, Pearson JL, Tsukayama KK, et al. Functional genomic analyses of mendelian and sporadic disease identify impaired eIF2α signaling as a generalizable mechanism for dystonia. Neuron. 2016;92(6):1238–51. https://doi.org/10.1016/j.neuron.2016.11.012This manuscript shows that defective eIF2α pathway has a common mechanism shared by TOR1A- and PRKRA-related dystonias.
Mencacci NE, Reynolds R, Ruiz SG, Vandrovcova J, Forabosco P, Sánchez-Ferrer A, et al. Dystonia genes functionally converge in specific neurons and share neurobiology with psychiatric disorders. 2020;Brain, 143(9):2771–87. https://doi.org/10.1093/brain/awaa217This paper shows that genes underlying different dystonia phenotypes are in fact part of shared networks involved in synaptic transmission. These findings corroborate the concept that categorizing dystonia based on the clinical subtype may not reflect the underlying biologic pathway.
Kumar KR, Davis RL, Tchan MC, Wali GM, Mahant N, Ng K, et al. Whole genome sequencing for the genetic diagnosis of heterogenous dystonia phenotypes. Parkinsonism Relat Disord. 2019;69:111–8. https://doi.org/10.1016/j.parkreldis.2019.11.004Kumar et al. demonstrate the advantages of whole-genome over whole-exome sequencing in dystonia, especially due to the detection of copy number variants.
Wirth T, Tranchant C, Drouot N, Keren B, Mignot C, Cif L, et al. Increased diagnostic yield in complex dystonia through exome sequencing. Parkinsonism Relat Disord. 2020;74:50–6. https://doi.org/10.1016/j.parkreldis.2020.04.003This paper highlights the superiority of whole-exome sequencing compared to gene panels in the diagnosis of monogenic dystonias, particularly when combined with neurodevelopmental delay.
Funding
NEM is funded by a Parkinson’s foundation grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Movement Disorders
Rights and permissions
About this article
Cite this article
Keller Sarmiento, I.J., Mencacci, N.E. Genetic Dystonias: Update on Classification and New Genetic Discoveries. Curr Neurol Neurosci Rep 21, 8 (2021). https://doi.org/10.1007/s11910-021-01095-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11910-021-01095-1