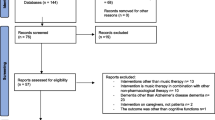
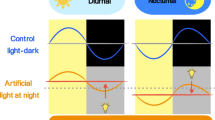
Abstract
Alzheimer’s disease (AD) is increasing in prevalence and has a significant impact on caregivers and the healthcare system. One of the many physiologic process affected by AD is the circadian system, with disruption reflected in abnormalities of the sleep-wake cycle. This interaction is bidirectional, with circadian and sleep disruption influencing disease progression. Understanding the bidirectional relationship between AD and circadian disruption may allow for earlier recognition of the potential to develop dementia as well as improved targeted approaches for therapy. Therapies including melatonin and bright light therapy may be advantageous in improving sleep and circadian rhythms and preventing the progression of disease. However, unfortunately, these modalities are not curative, and additional research is needed to improve treatment options for these individuals.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Urrestarazu E, Iriarte J. Clinical management of sleep disturbances in Alzheimer’s disease: current and emerging strategies. Nature and science of sleep. 2016;8:21–33.
Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–83.
Pistacchi M, Gioulis M, Contin F, Sanson F, Marsala SZ. Sleep disturbance and cognitive disorder: epidemiological analysis in a cohort of 263 patients. Neurological Sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2014;35:1955–62.
Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6:347–52.
Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics: the official journal of the Japanese Psychogeriatric Society. 2015;15:65–74.
Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. International Journal of Geriatric Psychiatry. 1998;13:682–90.
Slats D, Claassen JA, Verbeek MM, Overeem S. Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: focus on the role of hypocretin and melatonin. Ageing Res Rev. 2013;12:188–200.
Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68.
Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41.
Cassone VM, Chesworth MJ, Armstrong SM. Entrainment of rat circadian rhythms by daily injection of melatonin depends upon the hypothalamic suprachiasmatic nuclei. Physiol Behav. 1986;36:1111–21.
Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313.
Mattis J, Sehgal A. Circadian rhythms, sleep, and disorders of aging. Trends in Endocrinology and Metabolism: TEM. 2016;27:192–203.
Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–41.
Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science (New York, NY). 1996;271:216–9.
de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7.
Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci Off J Soc Neurosci. 2005;25:6716–20.
Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4.
Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci Off J Soc Neurosci. 2009;29:10939–49.
Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33.
Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci Off J Soc Neurosci. 1998;18:9996–10015.
Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63.
Gerdin MJ, Masana MI, Rivera-Bermudez MA, et al. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB Journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1646–56.
Tzischinsky O, Shlitner A, Lavie P. The association between the nocturnal sleep gate and nocturnal onset of urinary 6-sulfatoxymelatonin. J Biol Rhythm. 1993;8:199–209.
Hut RA, Van der Zee EA. The cholinergic system, circadian rhythmicity, and time memory. Behav Brain Res. 2011;221:466–80.
Yamakawa GR, Basu P, Cortese F, et al. The cholinergic forebrain arousal system acts directly on the circadian pacemaker. Proc Natl Acad Sci U S A. 2016;113:13498–503.
Abbott SM, Arnold JM, Chang Q, et al. Signals from the brainstem sleep/wake centers regulate behavioral timing via the circadian clock. PLoS One. 2013;8:e70481.
Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA neurology. 2014;71:505–8.
Abbott SM, Zee PC. Irregular sleep-wake rhythm disorder. Sleep medicine clinics. 2015;10:517–22.
Merlino G, Piani A, Gigli GL, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010;11:372–7.
Craig LA, McDonald RJ. Chronic disruption of circadian rhythms impairs hippocampal memory in the rat. Brain Res Bull. 2008;76:141–51.
Stopa EG, Volicer L, Kuo-Leblanc V, et al. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J Neuropathol Exp Neurol. 1999;58:29–39.
Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16:571–6.
Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44.
Wang JL, Lim AS, Chiang WY, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol. 2015;78:317–22.
Liu RY, Zhou JN, Hoogendijk WJ, et al. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J Neuropathol Exp Neurol. 2000;59:314–22.
Coogan AN, Schutova B, Husung S, et al. The circadian system in Alzheimer’s disease: disturbances, mechanisms, and opportunities. Biol Psychiatry. 2013;74:333–9.
Tate B, Aboody-Guterman KS, Morris AM, Walcott EC, Majocha RE, Marotta CA. Disruption of circadian regulation by brain grafts that overexpress Alzheimer beta/A4 amyloid. Proc Natl Acad Sci U S A. 1992;89:7090–4.
Song Q, Feng G, Huang Z, Chen X, Chen Z, Ping Y. Aberrant axonal arborization of PDF neurons induced by Abeta42-mediated JNK activation underlies sleep disturbance in an Alzheimer’s model. Mol Neurobiol. 2016.
Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurology. 2013;70:587–93.
Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9.
Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurology. 2013;70:1537–43.
Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra122.
Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cyclee. Science (New York, NY) 2009; 326:1005–1007.
Selkoe DJ. The therapeutics of Alzheimer’s disease: where we stand and where we are heading. Ann Neurol. 2013;74:328–36.
Huang Y, Potter R, Sigurdson W, et al. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol. 2012;69:51–8.
Tabuchi M, Lone SR, Liu S, et al. Sleep interacts with abeta to modulate intrinsic neuronal excitability. Current biology: CB. 2015;25:702–12.
Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148.
Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003;37:925–37.
Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–22.
Bero AW, Yan P, Roh JH, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–6.
Nir Y, Staba RJ, Andrillon T, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–69.
Martin PR, Loewenstein RJ, Kaye WH, Ebert MH, Weingartner H, Gillin JC. Sleep EEG in Korsakoff's psychosis and Alzheimer’s disease. Neurology. 1986;36:411–4.
Song H, Moon M, Choe HK, et al. Abeta-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol Neurodegener. 2015;10:13.
Varga AW, Wohlleber ME, Gimenez S, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Abeta42 levels in cognitively normal elderly. Sleep. 2016;39:2041–8.
Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science (New York, NY). 2013;342:373–7.
Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science (New York, NY). 1995;269:973–7.
Belanger V, Picard N, Cermakian N. The circadian regulation of Presenilin-2 gene expression. Chronobiol Int. 2006;23:747–66.
Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–21.
Wu YH, Swaab DF. The human pineal gland and melatonin in aging and Alzheimer’s disease. J Pineal Res. 2005;38:145–52.
Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res. 2003;35:125–30.
Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon 4/4 genotype. J Clin Endocrinol Metab. 1999;84:323–7.
Lin L, Huang QX, Yang SS, Chu J, Wang JZ, Tian Q. Melatonin in Alzheimer’s disease. Int J Mol Sci. 2013;14:14575–93.
Colwell CS, Kaufman CM, Menaker M. Phase-shifting mechanisms in the mammalian circadian system: new light on the carbachol paradox. J Neurosci Off J Soc Neurosci. 1993;13:1454–9.
Erhardt C, Galani R, Jeltsch H, et al. Modulation of photic resetting in rats by lesions of projections to the suprachiasmatic nuclei expressing p75 neurotrophin receptor. Eur J Neurosci. 2004;19:1773–88.
Feng R, Li L, Yu H, Liu M, Zhao W. Melanopsin retinal ganglion cell loss and circadian dysfunction in Alzheimer’s disease (review). Mol Med Rep. 2016;13:3397–400.
La Morgia C, Ross-Cisneros FN, Koronyo Y, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79:90–109.
Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015;277:513–27.
Maury E, Hong HK, Bass J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes & Metabolism. 2014;40:338–46.
Weljie AM, Meerlo P, Goel N, et al. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc Natl Acad Sci U S A. 2015;112:2569–74.
Davies SK, Ang JE, Revell VL, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A. 2014;111:10761–6.
Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62.
Forrestel AC, Miedlich SU, Yurcheshen M, Wittlin SD, Sellix MT. Chronomedicine and type 2 diabetes: shining some light on melatonin. Diabetologia. 2016.
Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA neurology. 2015;72:1013–20.
Luciano R, Barraco GM, Muraca M, et al. Biomarkers of Alzheimer disease, insulin resistance, and obesity in childhood. Pediatrics. 2015;135:1074–81.
Alessi CA, Yoon EJ, Schnelle JF, Al-Samarrai NR, Cruise PA. A randomized trial of a combined physical activity and environmental intervention in nursing home residents: do sleep and agitation improve? J Am Geriatr Soc. 1999;47:784–91.
Graf A, Wallner C, Schubert V, et al. The effects of light therapy on mini-mental state examination scores in demented patients. Biol Psychiatry. 2001;50:725–7.
Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. International Journal of Geriatric Psychiatry. 1999;14:520–5.
Dowling GA, Graf CL, Hubbard EM, Luxenberg JS. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. West J Nurs Res. 2007;29:961–75.
• van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62. A recent systematic review and meta-analysis looking at the effects of light therapy in circadian rhythm sleep disorders, insomnia, AD, and dementia. Eleven studies included patients with AD/dementia and a total of N = 211 patients, demonstrated significant effects of light therapy in patients with AD/dementia in improving sleep onset latency, total sleep time, time in bed, sleep efficiency, and sleep quality. However, there was no significant improvement seen in wake after sleep onset or early morning awakenings.
Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2014: Cd003946
Rudnitskaya EA, Muraleva NA, Maksimova KY, Kiseleva E, Kolosova NG, Stefanova NA. Melatonin attenuates memory impairment, amyloid-beta accumulation, and neurodegeneration in a rat model of sporadic Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD. 2015;47:103–16.
Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. Journal of Nippon Medical School = Nippon Ika Daigaku zasshi. 2003;70:334–41.
Wade AG, Farmer M, Harari G, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. 2014;9:947–61.
• Wang YY, Zheng W, Ng CH, Ungvari GS, Wei W, Xiang YT. Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2017;32:50–7. A recent meta-analysis looking at the effects of melatonin in AD. Seven studies included patients with AD and a total of N = 462 patients, with the administration of melatonin dosage ranging from 1.5 to 10 mg, from 10 days to 24 weeks, demonstrated that patients receiving melatonin treatment showed prolonged total sleep time at night. Melatonin, however, did not improve cognitive abilities assessed by the mini-mental state examination (MMSE) or the Alzheimer’s Disease Assessment Cognitive Subscale (ADACS).
Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging. 2007;28:1239–47.
Dowling GA, Burr RL, Van Someren EJ, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:239–46.
Furuya M, Miyaoka T, Yasuda H, et al. Marked improvement in delirium with ramelteon: five case reports. Psychogeriatrics: the official journal of the Japanese Psychogeriatric Society. 2012;12:259–62.
Ooms S, Ju YE. Treatment of sleep disorders in dementia. Curr Treat Options Neurol. 2016;18:40.
Camargos EF, Louzada LL, Quintas JL, Naves JO, Louzada FM, Nobrega OT. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double-blind, and placebo-controlled study. The American Journal of Geriatric Psychiatry: official journal of the American Association for Geriatric Psychiatry. 2014;22:1565–74.
Grippe TC, Goncalves BS, Louzada LL, et al. Circadian rhythm in Alzheimer disease after trazodone use. Chronobiol Int. 2015;32:1311–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yumna Saeed and Sabra M. Abbott declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sleep
Rights and permissions
About this article
Cite this article
Saeed, Y., Abbott, S.M... Circadian Disruption Associated with Alzheimer’s Disease. Curr Neurol Neurosci Rep 17, 29 (2017). https://doi.org/10.1007/s11910-017-0745-y
Published:
DOI: https://doi.org/10.1007/s11910-017-0745-y