Abstract
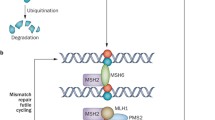
Prognosis for patients with glioblastoma continues to be limited, despite an aggressive, multimodal treatment including alkylating chemotherapy. Temozolomide, the most widely used alkylating agent in glioblastoma, is cytotoxic to cells by inducing DNA damage but can be rapidly repaired by the protein O 6-methylguanine DNA methyltransferase (MGMT). In a subset of glioblastomas, the MGMT promoter is methylated, impairing the repair mechanism and conferring chemosensitivity. However, MGMT is overexpressed in 60 % of glioblastomas providing an inherent resistance to alkylating agents and challenging the role of temozolomide in this population. This article reviews the data establishing MGMT promoter methylation as a prognostic factor in glioblastoma and its potential role as a predictor of temozolomide response. It focuses on results from recent studies in newly diagnosed glioblastoma, and the role of temozolomide in MGMT-unmethylated patients. We then turn the discussion to alternatives to temozolomide for newly diagnosed patients as well as therapeutic options at the time of recurrence.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlights as: • Of importance •• Of major importance
CBTRUS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2008. http://www.cbtrusorg. 2012 [updated 2012; cited 14 Aug 2014].
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–4.
Hegi ME, Diserens AC, Godard S, Dietrich PY, Regli L, Ostermann S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–4.
Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23(1):35–61.
Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2(9):552–60.
Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–93.
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003.
Liu L, Markowitz S, Gerson SL. Mismatch repair mutations override alkyltransferase in conferring resistance to temozolomide but not to 1,3-bis(2-chloroethyl)nitrosourea. Cancer Res. 1996;56(23):5375–9.
Pegg AE. Repair of O(6)-alkylguanine by alkyltransferases. Mutat Res. 2000;462(2–3):83–100.
Hotta T, Saito Y, Fujita H, Mikami T, Kurisu K, Kiya K, et al. O6-Alkylguanine-DNA alkyltransferase activity of human malignant glioma and its clinical implications. J Neurooncol. 1994;21(2):135–40.
Belanich M, Pastor M, Randall T, Guerra D, Kibitel J, Alas L, et al. Retrospective study of the correlation between the DNA repair protein alkyltransferase and survival of brain tumor patients treated with carmustine. Cancer Res. 1996;56(4):783–8.
Jaeckle KA, Eyre HJ, Townsend JJ, Schulman S, Knudson HM, Belanich M, et al. Correlation of tumor O6 methylguanine-DNA methyltransferase levels with survival of malignant astrocytoma patients treated with bis-chloroethylnitrosourea: a Southwest Oncology Group study. J Clin Oncol. 1998;16(10):3310–5.
Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4(1):1–6.
Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21(35):5427–40.
Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51.
Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793–7.
Vlassenbroeck I, Califice S, Diserens AC, Migliavacca E, Straub J, Di Stefano I, et al. Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. J Mol Diagn. 2008;10(4):332–7.
Everhard S, Tost J, El Abdalaoui H, Criniere E, Busato F, Marie Y, et al. Identification of regions correlating MGMT promoter methylation and gene expression in glioblastomas. Neuro Oncol. 2009;11(4):348–56.
Sciuscio D, Diserens AC, van Dommelen K, Martinet D, Jones G, Janzer RC, et al. Extent and patterns of MGMT promoter methylation in glioblastoma- and respective glioblastoma-derived spheres. Clin Cancer Res. 2011;17(2):255–66.
Moen EL, Stark AL, Zhang W, Dolan ME, Godley LA. The role of gene body cytosine modifications in MGMT expression and sensitivity to temozolomide. Mol Cancer Ther. 2014;13(5):1334–44.
Wick W, Weller M, van den Bent M, Sanson M, Weiler M, von Deimling A, et al. MGMT testing-the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372–85. Wick et al. thoroughly review recent data on the relationship of MGMT methylation status as a biomarker.
Hart MG, Garside R, Rogers G, Stein K, Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2013;4, CD007415.
Gallego Perez-Larraya J, Ducray F, Chinot O, Catry-Thomas I, Taillandier L, Guillamo JS, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–5.
Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115(15):3512–8.
Reifenberger G, Hentschel B, Felsberg J, Schackert G, Simon M, Schnell O, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131(6):1342–50. Reifenberger et al. report on a large, prospective, observational trial of elderly GBM patients and demonstrate no added benefit of adding temozolomide to patients with unmethylated MGMT promoter.
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–15. Wick et al. conducted a large study of elderly patients with high-grade glioma and found temozolomide monotherapy to be non-inferior to radiation in MGMT-methylated patients.
Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–26. Malmostrom et al. conducted a large, randomized trial of elderly patients with GBM and found no benefit of adding temozolomide to MGMT-unmethylated patients.
Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–8.
Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–403. Weller et al. outline European guidelines for the treatment of high-grade gliomas, including MGMT-unmethylated GBM.
Nabors L, Fink K, Mikkelsen T, Grujicic D, Tarnawski R, Nam D, et al. A randomized phase II study investigating cilengitide added to standard chemoradiotherapy in patients with newly diagnosed glioblastoma with unmethylated O6-methylguanine-DNA methyltransferase (MGMT) gene promoter: initial report of the CORE study. Eur J Cancer. 2013;49 Suppl 3:S17–8.
Stupp R, Hegi ME, Gorlia T, Erridge S, Grujicic D, Steinbach JP, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma and methylated O6-methylguanine-DNA methyltransferase (MGMT) gene promoter: key results of the multicenter, randomized, open-label, controlled, phase III CENTRIC study. J Clin Oncol. 2013;31 Suppl 3:2009.
Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(16):2712–8.
Herrlinger U, Schaefer N, Steinbach JP, Weyerbrock A, Hau P, Goldbrunner R, et al. Survival and quality of life in the randomized, multicenter GLARIUS trial investigating bevacizumab/irinotecan versus standard temozolomide in newly diagnosed, MGMT-non-methylated glioblastoma patients. J Clin Oncol. 2014;32 Suppl 5:2042.
Wick W, Gorlia T, van den Bent MJ, Vecht CJ, Steuve J, Brandes AA, et al. Radiation therapy and concurrent plus adjuvant temsirolimus (CCI-779) versus chemo-irradiation with temozolomide in newly diagnosed glioblastoma without methylation of the MGMT gene promoter. J Clin Oncol. 2014;32 Suppl 5:2003.
Wick W, Steinbach JP, Platten M, Hartmann C, Wenz F, von Deimling A, et al. Enzastaurin before and concomitant with radiation therapy, followed by enzastaurin maintenance therapy, in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation. Neuro Oncol. 2013;15(10):1405–12.
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–91. Gilbert et al. found no benefit to treatment with dose-dense temozolomide compared to conventional dosing for newly diagnosed GBM, regardless of MGMT promoter methylation status.
Perry JR, Belanger K, Mason WP, Fulton D, Kavan P, Easaw J, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–7.
Wick A, Felsberg J, Steinbach JP, Herrlinger U, Platten M, Blaschke B, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25(22):3357–61.
Norden AD, Lesser GJ, Drappatz J, Ligon KL, Hammond SN, Lee EQ, et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013;15(7):930–5.
Tabatabai G, Wick W, Steinbach JP, Wick A, Schnell O, Hau P et al. MGMT promoter methylation as a prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: first results from the randomized phase II DIRECTOR trial. J Clin Oncol. 2014;32(5s):(supple; abstr 2015).
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–53. Taal et al. reported an improved PFS9 in recurrent GBM treated with lomustine/bevacizumab compared to bevacizumab alone, regarding of MGMT promoter methylation status.
Compliance with Ethics Guidelines
Conflict of Interest
Jennie W. Taylor declare no conflict of interest.
David Schiff has received paid travel and accommodations from Merck to speak in a symposium about glioma.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuro-Oncology
Rights and permissions
About this article
Cite this article
Taylor, J.W., Schiff, D. Treatment Considerations for MGMT-Unmethylated Glioblastoma. Curr Neurol Neurosci Rep 15, 507 (2015). https://doi.org/10.1007/s11910-014-0507-z
Published:
DOI: https://doi.org/10.1007/s11910-014-0507-z