Abstract
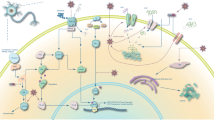
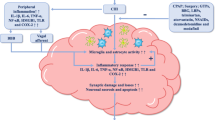
Sleepiness has long been recognized as a presenting symptom in obstructive sleep apnea syndrome, but persistent neurocognitive injury from sleep apnea has been appreciated only recently. Although therapy for sleep apnea markedly improves daytime symptoms, cognitive impairments may persist despite long-term therapy with continuous positive airway pressure. We know now that certain groups of neurons, typically those that are more metabolically active, are more vulnerable to injury than others. Animal models of sleep apnea oxygenation patterns have been instrumental in elucidating mechanisms of injury. The hypoxia/reoxygenation events result in oxidative, inflammatory, and endoplasmic reticulum stress responses in susceptible neural groups. With molecular pathways being fleshed out in animal models, it is time to carefully and systematically examine neural injury in humans and test the applicability of findings from animal models. To succeed, however, we cannot view sleep apnea as an isolated process. Rather, injury in sleep apnea is more likely the consequence of overlapping injuries from comorbid conditions. The progress in elucidating mechanisms of neural injury is palpable, and it now seems we indeed are closer to developing therapies to prevent and treat neural injury in obstructive sleep apnea.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Young T, Palta M, Dempsey J, et al.: The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993, 328:1230–1235.
Guilleminault C, Lee JH, Chan A: Pediatric obstructive sleep apnea syndrome. Arch Pediatr Adolesc Med 2005, 159:775–785.
Kales A, Caldwell AB, Cadieux RJ, et al.: Severe obstructive sleep apnea—II: associated psychopathology and psychosocial consequences. J Chronic Dis 1985, 38:427–434.
Findley LJ, Barth JT, Powers DC, et al.: Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest 1986, 90:686–690.
Powers CR, Frey WC: Maintenance of wakefulness test in military personnel with upper airway resistance syndrome and mild to moderate obstructive sleep apnea. Sleep Breath 2009, 13:253–258.
Archbold KH, Giordani B, Ruzicka DL, Chervin RD: Cognitive executive dysfunction in children with mild sleep-disordered breathing. Biol Res Nurs 2004, 5:168–176.
Montplaisir J, Bedard MA, Richer F, Rouleau I: Neurobehavioral manifestations in obstructive sleep apnea syndrome before and after treatment with continuous positive airway pressure. Sleep 1992, 15:S17–S19.
Bedard MA, Montplaisir J, Malo J, et al.: Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treatment with continuous positive airways pressure (CPAP). J Clin Exp Neuropsychol 1993, 15:330–341.
Engleman HM, Martin SE, Deary IJ, Douglas NJ: Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994, 343:572–575.
Engleman H, Joffe D: Neuropsychological function in obstructive sleep apnoea. Sleep Med Rev 1999, 3:59–78.
Henke KG, Grady JJ, Kuna ST: Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea-hypopnea syndrome. A randomized, placebo-controlled trial. Am J Respir Crit Care Med 2001, 163:911–917.
Lim W, Bardwell WA, Loredo JS, et al.: Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo-controlled study. J Clin Sleep Med 2007, 3:380–386.
Sangal RB, Sangal JM: Abnormal visual P300 latency in obstructive sleep apnea does not change acutely upon treatment with CPAP. Sleep 1997, 20:702–704.
Kotterba S, Rasche K, Widdig W, et al.: Neuropsychological investigations and event-related potentials in obstructive sleep apnea syndrome before and during CPAP-therapy. J Neurol Sci 1998, 159:45–50.
Inoue Y, Nanba K, Kojima K, et al.: P300 abnormalities in patients with severe sleep apnea syndrome. Psychiatry Clin Neurosci 2001, 55:247–248.
Mayer P, Dematteis M, Pepin JL, et al.: Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med 1999, 159:213–219.
Boyd JH, Petrof BJ, Hamid Q, et al.: Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med 2004, 170:541–546.
Mojon DS, Hedges TR 3rd, Ehrenberg B, et al.: Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol 2002, 120:601–605.
Palombi K, Renard E, Levy P, et al.: Non-arteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoea. Br J Ophthalmol 2006, 90:879–882.
Dziewas R, Schilling M, Engel P, et al.: Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry 2007, 78:295–297.
Fanfulla F, Malaguti S, Montagna T, et al.: Erectile dysfunction in men with obstructive sleep apnea: an early sign of nerve involvement. Sleep 2000, 23:775–781.
Aloia MS, Sweet LH, Jerskey BA, et al.: Treatment effects on brain activity during a working memory task in obstructive sleep apnea. J Sleep Res 2009 Sep 17 (Epub ahead of print).
Archbold KH, Borghesani PR, Mahurin RK, et al.: Neural activation patterns during working memory tasks and OSA disease severity: preliminary findings. J Clin Sleep Med 2009, 5:21–27.
Macey PM, Henderson LA, Macey KE, et al.: Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 2002, 166:1382–1387.
Morrell MJ, McRobbie DW, Quest RA, et al.: Changes in brain morphology associated with obstructive sleep apnea. Sleep Med 2003, 4:451–454.
Bartlett DJ, Rae C, Thompson CH, et al.: Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Med 2004, 5:593–596.
Halbower AC, Degaonkar M, Barker PB, et al.: Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med 2006, 3:e301.
Castronovo V, Canessa N, Strambi LF, et al.: Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep 2009, 32:1161–1172.
Ayalon L, Ancoli-Israel S, Drummond SP: Altered brain activation during response inhibition in obstructive sleep apnea. J Sleep Res 2009, 18:204–208.
Yaouhi K, Bertran F, Clochon P, et al.: A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res 2009, 18:36–48.
Cross RL, Kumar R, Macey PM, et al.: Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep 2008, 31:1103–1109.
Kumar R, Macey PM, Cross RL, et al.: Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety 2009, 26:480–491.
Macey PM, Harper RM: OSA brain morphology differences: magnitude of loss approximates age-related effects. Am J Respir Crit Care Med 2005, 172:1056–1057; author reply 1057–1058.
Thomas RJ, Rosen BR, Stern CE, et al.: Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol 2005, 98:2226–2234.
Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE: Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychosom Med 2001, 63:579–584.
Felver-Gant JC, Bruce AS, Zimmerman M, et al.: Working memory in obstructive sleep apnea: construct validity and treatment effects. J Clin Sleep Med 2007, 3:589–594.
Aloia MS, Ilniczky N, Di Dio P, et al.: Neuropsychological changes and treatment compliance in older adults with sleep apnea. J Psychosom Res 2003, 54:71–76.
Tonon C, Vetrugno R, Lodi R, et al.: Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep 2007, 30:305–311.
Antczak J, Popp R, Hajak G, et al.: Positron emission tomography findings in obstructive sleep apnea patients with residual sleepiness treated with continuous positive airway pressure. J Physiol Pharmacol 2007, 58(Suppl 5): 25–35.
Gozal D, Daniel JM, Dohanich GP: Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 2001, 21:2442–2450.
Li RC, Row BW, Kheirandish L, et al.: Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis 2004, 17:44–53.
Row BW, Kheirandish L, Li RC, et al.: Platelet-activating factor receptor-deficient mice are protected from experimental sleep apnea-induced learning deficits. J Neurochem 2004, 89:189–196.
Gozal D, Row BW, Kheirandish L, et al.: Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem 2003, 86:1545–1552.
Goldbart AD, Row BW, Kheirandish-Gozal L, et al.: High fat/refined carbohydrate diet enhances the susceptibility to spatial learning deficits in rats exposed to intermittent hypoxia. Brain Res 2006, 1090:190–196.
Row BW: Intermittent hypoxia and cognitive function: implications from chronic animal models. Adv Exp Med Biol 2007, 618:51–67.
Row BW, Liu R, Xu W, et al.: Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 2003, 167:1548–1553.
Kheirandish L, Gozal D, Pequignot JM, et al.: Intermittent hypoxia during development induces long-term alterations in spatial working memory, monoamines, and dendritic branching in rat frontal cortex. Pediatr Res 2005, 58:594–599.
Perry JC, D'Almeida V, Lima MM, et al.: Intermittent hypoxia and sleep restriction: motor, cognitive and neurochemical alterations in rats. Behav Brain Res 2008, 189:373–380.
Zhang JX, Lu XJ, Wang XC, et al.: Intermittent hypoxia impairs performance of adult mice in the two-way shuttle box but not in the Morris water maze. J Neurosci Res 2006, 84:228–235.
Veasey SC, Davis CW, Fenik P, et al.: Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004, 27:194–201.
• Zhu Y, Fenik P, Zhan G, et al.: Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci 2007, 27:10060–10071. This study establishes that intermittent hypoxia irreversibly impairs wake neurons and results in a loss of neurons, supporting the concept that sleep apnea may contribute to neurodegeneration.
Zhan G, Serrano F, Fenik P, et al.: NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 2005, 172:921–929.
Zhu Y, Fenik P, Zhan G, et al.: Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J Neurosci 2008, 28:2168–2178.
Acknowledgment
This work was supported in part by National Institutes of Health grants HL080492 and HL079555.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, D.C., Veasey, S.C. Neural Injury in Sleep Apnea. Curr Neurol Neurosci Rep 10, 47–52 (2010). https://doi.org/10.1007/s11910-009-0078-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-009-0078-6