Abstract
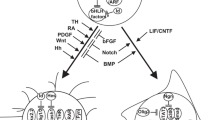
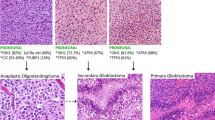
Transformation of a normal cell into a malignant cell involves a series of events that damage the genome. Gliomas are the most common adult neoplasm of the central nervous system. To develop new therapeutic strategies requires an understanding of the specific lesions that occur and contribute to this malignant process. Initially, data reported from the analyses of human gliomas were quite variable. This has recently changed as more data have become available and the selection of tissue analyzed is coupled with clinical criteria. Specific genetic lesions are now defining different glioma pathways, and some aberrations may be indicative of therapeutic response. This review focuses on the specific genetic aberrations associated with astrocytic and oligodenroglial tumors.
Similar content being viewed by others
References and Recommended Reading
Shapiro WR, Shapiro JR, Walker RW: Central nervous system. In Clinical Oncology. Edited by Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE. New York: Churchill Livingstone; 2000:1103–1192. This article characterizes in detail the genetic changes identified in gliomas. No distinction is made between primary and secondary gliomas.
James CD, Carlbom E, Dumanski JP, et al.: Clonal genomic alterations in glioma malignancy stages. Cancer Res 1988, 48:5546–5551.
Kleihues P, Lübbe J, Watanabe K, et al.: Genetic alterations associated with glioma progression. Verh Dtsch Ges Path 1995, 78:43–47.
von Deimling A, von Ammon K, Schoenfeld D, et al.: Subsets of glioblastomas multiforme defined by molecular genetic analysis. Brain Pathol 1993, 3:19–26.
Kleihues P, Ohgaki H: Primary and secondary glioblastoma: from concept to clinical diagnosis. Neuro Oncol 2000, 1:44–51.
Hollstein M, Sidransky D, Vogelstein B, et al.: p53 mutations in human cancers. Science 1991, 253:49–53.
de Fromentel CC, Soussi T: TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer 1992, 4:1–15.
Lang FF, Miller DC, Koslow M, et al.: Pathways leading to glioblastoma multiforme: a molecular analysis of genetic alterations in 65 astrocytic tumors. J Neurosurg 1994, 81:427–436.
Watanabe K, Sato K, Biernat W, et al.: Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res 1997, 3:523–530.
Kirsch M, Wilson JC, Black P: Platelet-derived growth factor in human brain tumors. J Neurooncol 1997, 35:289–302.
Nistér M, Claesson-Welsh L, Eriksson A, et al.: Differential expression of platelet-derived growth factor receptors in human malignant glioma cell lines. J Biol Chem 1991, 266:16755–16763.
Bigner SH, Mark J, Mahaley MS, et al.: Patterns of the early, gross chromosomal changes in malignant human gliomas. Hereditas 1984, 101:103–113.
Scheck AC, Mehta BM, Beikman MK, et al.: BCNU-resistant human glioma cells with over-representation of chromosomes 7 and 22 demonstrate increased copy number and expression of platelet-derived growth factor genes. Genes Chromosomes Cancer 1993, 8:137–148.
Cavenee WK, Furnari FB, Nagane M, et al.: Astrocytic tumours. In Pathology & Genetics. Tumours of the Nervous System. Edited by Kleihues P, Cavenee WK. Lyon, France: IARC Press; 2000:9–54. Excellent review of pathology and molecular genetic changes associated with all central nervous system tumors.
Hoang-Xuan K, Merel P, Vega F, et al.: Analysis of the NF2 tumor-suppressor gene and of chromosome 22 deletions in gliomas. Int J Cancer 1995, 60:478–481.
Bigner SH, Mark J, Bigner DD: Cytogenetics of human brain tumors. Cancer Genet Cytogenet 1990, 47:141–154.
Shapiro JR, Scheck AC: Brain tumors. In Cytogenetic Markers of Human Cancer. Edited by Wolman SR, Sell S. New Jersey: The Humana Press; 1997:319–368.
He J, Olson JJ, James CD: Lack of p16INK4 or retinoblastoma protein pRb, or amplification-associated overexpression of cdk4 is observed in distinct subsets of malignant glial tumors and cell lines. Cancer Res 1995, 55:4833–4836.
Dirks PB, Rutka JT: Current concepts in neuro-oncology: the cell cycle-a review. Neurosurg 1997, 40:1000–1015.
Nakamura M, Yang F, Fujisawa H, et al.: Loss of heterozygosity on chromosome 19 in secondary glioblastomas. J Neuropathol Exp Neurol 2000, 596:539–543.
Fults D, Pedone C: Deletion mapping of the long arm of chromosome 10 in glioblastoma multiforme. Genes Chromosomes Cancer 1993, 7:173–177.
Lang FF, Miller DC, Koslow M, et al.: Pathways leading to glioblastoma multiforme: a molecular analysis of genetic alterations in 65 astrocytic tumors. J Neurosurg 1994, 81:427–436.
Steck PA, Pershouse MA, Jasser SA, et al.: Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genet 1997, 15:356–362.
Tohma Y, Gratas C, Biernat W, et al.: PTEN mutations are frequent in primary glioblastomas de novo. but not in secondary glioblastomas. J Neuropath Exp Neurol 1998, 57:684–689.
Mollenhauer J, Wiemann S, Scheurlen W, et al.: DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3-26.1 is deleted in malignant brain tumours. Nature Genet 1997, 171:32–39.
Somerville RP, Shoshan Y, Eng C, et al.: Molecular analysis of two putative tumour suppressor genes, PTEN and DMBT, which have been implicated in glioblastoma multiforme disease progression. Oncogene 1998, 17:1755–1757.
Guha A, Dasher K, McL.Black P, et al.: Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer 1995, 60:168–173.
Harsh GR, Keating MT, Escobedo JA, et al.: Platelet derived growth factor (PDGF) autocrine components in human tumor cell lines. J Neurooncol 1990, 1:1–12.
Hermanson M, Nistér M, Betsholtz C, et al.: Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor PDGF. B chain and PDGF receptor suggests autocrine growth stimulation. Proc Natl Acad Sci U S A 1988, 85:7748–7752.
Hermanson M, Funa K, Hartman M, et al.: Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 1992, 52:3213–3219.
Plare KH, Breier G, Farrell CL, et al.: Platelet-derived growth factor receptor-beta is induced during tumor development and upregulated during tumor progression in endothelial cells in human gliomas. Lab Invest 1992, 67:529–534.
Ekstrand BC, Bigner SH, Fearon ER: DCC gene mutations and loss of expression in glioblastomas. Proc Am Assoc Cancer Res 1995, 36:574–574.
Reyes-Mugica M, Rieger-Christ K, Ohgaki H, et al.: Loss of DCC expression and glioma progression. Cancer Res 1997, 573:382–386.
Scheck AC, Coons SW: Expression of the tumor suppressor gene DCC in human gliomas. Cancer Res 1993, 53:5605–5609.
Watanabe K, Tachibana O, Sato K, et al.: Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol 1996, 6:217–224.
Ekstrand AJ, Sugawa N, James CD, et al.: Amplified and rearranged EGFR genes in human glioblastomas reveal deletions of sequences encoding portions of the N-and/or C-terminal tails. Proc Natl Acad Sci U S A 1992, 89:4309–4313.
Tang P, Steck PA, Yung WK: The autocrine loop of TGF-a/EGFR and brain tumors. J Neurooncol 1997, 353:303–314.
Fujisawa H, Kurrer M, Reis RM, et al.: Acquisition of the glioblastoma phenotype during astrocytoma progression is associated with loss of heterozygosity on 10q25-qter. Am J Pathol 1999, 1552:387–394.
Reifenberger G, Liu L, Ichimura K, et al.: Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res 1993, 53:2736–2739.
Black P, Westphal M: Growth factors in brain tumors. J Neurooncol 1997, 353:193–372.
Reifenberger G, Kros JM, Burger PC, et al.: Oligodendroglial tumors. In Pathology and Genetics: Tumours of the Nervous System. Edited by Kleihues P, Cavenee WK: Lyon, France: IARC Press, 2000:55–70. Excellent review of the histopathology, characteristics, and molecular genetic abnormalities associated with oligodendroglial tumors.
Bello MJ, Leone PE, Nebreda P, et al.: Allelic status of chromosome 1 in neoplasms of the nervous system. Cancer Genet Cytogenet 1995, 83:160–164.
Ransom DT, Ritland SR, Kimmel DW, et al.: Cytogenetic and loss of heterozygosity studies in ependymomas, pilocytic astrocytomas and oligodendrogliomas. Genes Chromosomes Cancer 1992, 5:348–356.
Reifenberger J, Reifenberger G, Liu L, et al.: Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol 1994, 145:1175–1190.
von Deimling A, Louis DN, von Ammon K, et al.: Evidence for a tumor suppressor gene on chromosome 19q associated with human astrocytomas, oligodendrogliomas, and mixed gliomas. Cancer Res 1992, 52:4277–4279.
Yong WH, Chou D, Ueki K, et al.: Chromosome 19q deletions in human gliomas overlap telomeric to D19S219 and may target a 425 kb region centromeric to D19S112. J Neuropath Exp Neurol 1995, 54:622–626.
Bello MJ, Leone PE, Vaquero J, et al.: Allelic loss at 1p and 19q frequently occurs in association and may represent early oncogenic events in oligodendroglial tumors. Int J Cancer 1995, 643:207–210.
Kraus JA, Koopmann J, Kaskel P, et al.: Shared allelic losses on chromosomes 1p and 19q suggest a common origin of oligodendroglioma and oligoastrocytoma. J Neuropath Exp Neurol 1995, 54:91–95.
Bello MJ, Vaquero J, de Campos JM, et al.: Molecular analysis of chromosome 1 abnormalities in human gliomas reveals frequent loss of 1p in oligodendroglial tumors. Int J Cancer 1994, 57:172–175.
Husemann K, Wolter M, Buschges R, et al.: Identification of two distinct deleted regions on the short arm of chromosome 1 and rare mutation of the CDKN2C gene from 1p32 in oligodendroglial tumors. J Neuropathol Exp Neurol 1999, 5810:1041–1050.
Pohl U, Cairncross JG, Louis DN: Homozygous deletions of the CDKN2C/p18INK4C gene on the short arm of chromosome 1 in anaplastic oligodendrogliomas. Brain Pathol 1999, 9:639–643.
Mai M, Huang H, Reed C, et al.: Genomic organization and mutation analysis of p73 in oligodendrogliomas with chromosome 1p-arm deletions. Genomics 1998, 51:359–363.
Bigner SH, Matthews MR, Rasheed BK, et al.: Molecular genetic aspects of oligodendrogliomas including analysis by comparative genomic hybridization. Am J Pathol 1999, 1552:375–386. An excellent review of oligodendrogliomas, analyzed by comparing comparative genomic hybridization, cytogenetics, and molecular technologies.
Jeuken JW, Nelen MR, Vermeer H, et al.: PTEN mutation analysis in two genetic subtypes of high-grade oligodendroglial tumors. PTEN is only occasionally mutated in one of the two genetic subtypes. Cancer Genet Cytogenet 2000, 1191:42–47.
Kros JM, Godschalk JJ, Krishnadath KK, et al.: Expression of p53 in oligodendrogliomas. J Pathol 1993, 171:285–290.
Di Rocco F, Carroll RS, Zhang J, et al.: Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery 1998, 42:341–346.
Smith JS, Wang XY, Qian J, et al.: Amplification of the platelet-derived growth factor receptor-A PDGFRA. gene occurs in oligodendrogliomas with grade IV anaplastic features. J Neuropathol Exp Neurol 2000, 596:495–503.
Reifenberger G, Reifenberger J, Liu L, et al.: Molecular genetics of oligodendroglial tumors. In Brain Tumor Research and Therapy. Edited by Nagai M. Tokyo: Springer-Verlag; 1998:187–209.
Reifenberger J, Reifenberger G, Ichimura K, et al.: Epidermal growth factor receptor expression in oligodendroglial tumours. Am J Pathol 1996, 149:29–35.
Chan AS, Leung SY, Wong MP, et al.: Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol 1998, 227:816–826.
Nishikawa R, Cheng SY, Nagashima R, et al.: Expression of vascular endothelial growth factor in human brain tumors. Acta Neuropathol Berlin 1998, 965:453–462.
Pietsch T, Valter MM, Wolf HK, et al.: Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol Berlin 1997, 932:109–117.
Maintz D, Fiedler K, Koopmann J, et al.: Molecular genetic evidence for subtypes of oligoastrocytomas. Neuropathol Exp Neurol 1997, 5610:1098–1104.
Reifenberger J, Ring GU, Gies U, et al.: Analysis of p53 mutation and epidermal growth factor receptor amplification in recurrent gliomas with malignant progression. Neuropathol Exp Neurol 1996, 55:822–831.
Ino Y, Zlatescu MC, Sasaki H, et al.: Long survival and therapeutic responses in patients with histologically disparate high-grade gliomas demonstrating chromosome 1p loss. J Neurosurg 2000, 92:983–990.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shapiro, J.R. Genetics of brain neoplasms. Curr Neurol Neurosci Rep 1, 217–224 (2001). https://doi.org/10.1007/s11910-001-0021-y
Issue Date:
DOI: https://doi.org/10.1007/s11910-001-0021-y