Abstract
Vaccines based on attenuated or killed viruses and bacteria are highly effective in preventing infection with a range of pathogens, but can have safety issues. Therefore, a move is underway toward the development of subunit vaccines based on recombinant proteins or naked DNA. However, protein subunit vaccines are typically poorly immunogenic when administered alone and therefore require coadministration with adjuvants to boost the immune response. For many decades, very little progress was made in understanding the mechanism of action of adjuvants, but recently several significant breakthroughs have occurred in this area. The binding of pathogen-derived molecules to different immune sensors, including Toll-like receptors (TLR), nucleotide-binding oligomerization domain-like receptors (NLR), and retinoic acid–inducible gene (RIG)-1–like receptors (RLR), activates important innate immune pathways and provides not only an understanding of how current vaccines and adjuvants work, but also potential targets for novel adjuvant development.
Similar content being viewed by others
Introduction
Vaccination is an extremely powerful tool for preventing infectious diseases. Eradication of smallpox and the dramatic reduction in polio and measles throughout the world represent the most significant successes of vaccination. However, infectious diseases remain a leading cause of death worldwide. Traditional vaccines have mainly consisted of live attenuated pathogens, whole inactivated organisms, or inactivated bacterial toxins. The development of more advanced and effective vaccines to combat infectious diseases (eg, influenza, HIV, tuberculosis, and malaria) is a major goal of modern medical research. However, these new-generation vaccines are usually based on recombinant proteins, and although safer, are often less immunogenic than traditional attenuated or killed vaccines. To elicit a robust immune response, protein subunit vaccines typically need a boost from an adjuvant, a substance that derives its name from the Latin adjuvare, meaning “to help.” A successful vaccine, therefore, should contain not only protective antigen(s), but also a good adjuvant that can effectively amplify the protective immune responses.
Although alum and oil-in-water emulsions were used for decades as human vaccine adjuvants, their mechanism of action remained unclear. It was reported that these adjuvants acted by increasing antigen availability and uptake by immune cells, but their underlying molecular mode of action was not understood. Janeway [1] famously referred to adjuvants as the “immunologist’s dirty little secret.” Janeway’s hypothesis on the innate immune sensory system, and the intense research efforts invested into dissecting the molecular basis of innate immune activation, shed much light on how adjuvants shape the quality and quantity of the immune response to foreign antigens [2].
Induction of Protective Immunity by Vaccination
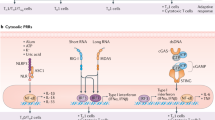
The induction of a protective immune response to a pathogen relies on a complex interplay of the cells and molecules of the innate and adaptive immune systems. Dendritic cells (DCs) play a crucial role in linking the innate and adaptive immune systems. DC and other innate immune cells express pattern-recognition receptors (PRRs) that have broad specificity capable of detecting common structural motifs or pathogen-associated molecular patterns (PAMPs) (Fig. 1). PAMPs derived from different classes of pathogens bind to diverse families of PRRs that include Toll-like receptors (TLRs), C-type lectin–like receptors (CLRs), retinoic acid-inducible gene (RIG)–like receptors (RLRs), and nucleotide-binding oligomerization domain (NOD)–like receptors (NLRs). These interactions decode pathogen information by triggering distinct signaling pathways to differentially activate antigen-presenting cells (APCs), thereby directing the adaptive effector response in a manner specifically tailored to the invading microbe [2]. The recently obtained wealth of information about the innate immune system has not only provided a greater appreciation of how existing adjuvants function but also a new set of targets for the rational development of novel adjuvants.
Mechanisms of innate immune activation induced by vaccine adjuvants. Pathogen-associated molecular patterns (PAMPs), which are components of many vaccines and adjuvants, activate cell surface-associated or intracellular Toll-like receptors (TLRs) or nucleotide-binding oligomerization domain-like receptors (NLRs). These pattern-recognition receptors then interact with specific adaptor molecules culminating in nuclear factor (NF)-κB or interferon (IFN) regulatory factor (IRF) activation. Viral-derived products (double-stranded or single-stranded RNA) can also activate endosomal TLRs, along with retinoic acid-inducible gene (RIG-1), which induces type I IFN production. Alum activates the cytosolic NACHT-, leucine-rich-repeat-, and pyrin-domain-containing protein 3 (NALP3) inflammasome leading to pro-interleukin (IL)-1β cleavage into bioactivated IL-1β. IPS—interferon-β promoter stimulator; MDA—melanoma differentiation factor; NOD—nucleotide-binding oligomerization domain; TNF—tumor necrosis factor
Vaccines based on live attenuated pathogens and inactivated whole pathogens have been extremely successful in preventing many common infectious diseases. The potent immunogenicity of such vaccines can be attributed to the presence of endogenous adjuvants, namely, the high content of PAMPs (eg, lipopolysaccharide [LPS], CpG-containing oligonucleotides [CpG], and peptidoglycans) [3, 4].
TLRs
TLRs represent a family of evolutionary conserved PRRs. TLRs are type I transmembrane receptors characterized by leucine-rich repeats (LRRs) in the extracellular portion and an intracellular Toll/interleukin (IL)-1 receptor (TIR) domain, which is homologous to the intracellular domain of IL-1 receptor family members [5]. Since their discovery, it was established that TLRs play important roles in recognizing specific microbial components derived from pathogens, including bacteria, fungi, protozoa, and viruses. Ten functional TLRs have been described in humans, many of which are widely expressed by different cell types of the immune system, including DC, macrophages, natural killer (NK) cells, mast cells, neutrophils, B cells, T cells, and nonimmune cells (e.g., fibroblasts and epithelial cells). TLRs can be divided into subfamilies primarily recognizing similar PAMPs; TLR1, TLR2, TLR4, and TLR6 recognize lipid structures, whereas TLR3, TLR7, TLR8, and TLR9 recognize nucleic acids. Most TLRs (TLR1, 2, 4, 5, 6, and 10) are expressed on the cell surface, whereas other TLRs (TLR3, 7, 8, and 9) are present within endosomal compartments. Furthermore, TLRs are expressed as homodimers or heterodimers (TLR2 + TLR1 or TLR2 + TLR6), and each TLR recognizes distinct microbial stimuli. TLRs also differ in their cellular distribution and the intracellular signals they trigger. All signaling pathways triggered by TLRs use the adaptor protein MyD88, with the exception of TLR3, which uses TIR-domain–containing adaptor-inducing interferon (IFN)-β (TRIF). LPS binding to TLR4 activates both MyD88 and TRIF.
The TLR4 agonist LPS was recognized as an adjuvant, capable of driving antibody responses to mixed protein antigens as early as 1955 [6], but is too toxic for use as an adjuvant for human vaccines. Monophosphoryl lipid A (MPL) is a chemically modified derivative of lipid A moiety of LPS and is considerably less toxic, but has similar immunostimulatory activity. MPL was the first TLR ligand approved for human use in the hepatitis B vaccine, Fendrix (GlaxoSmithKline Biologicals, Rixensart, Belgium) [7]. The formulation strategy used appears to be critical in directing the type of immune response elicited by MPL. Although MPL aqueous formulation enhances antibody responses, MPL in oil formulation is more effective at stimulating T-cell responses [8]. Many of the adjuvant systems developed by GlaxoSmithKline Biologicals incorporate MPL. AS02 is an oil-in-water emulsion containing MPL and QS-21 (a saponin-derived immunostimulator) that induces strong antibody and Th1 responses. AS02 is being evaluated in clinical trials in vaccines against malaria, human papillomavirus (HPV), HBV, tuberculosis, and HIV [9–12]. AS01 is a liposomal formulation containing MPL that induces potent humoral and cell-mediated responses, including cytotoxic T-lymphocyte responses, and is being evaluated in clinical trials of vaccine against malaria.
An exciting new development in the adjuvant field was the recent announcement that the US Food and Drug Administration (FDA) approved Cervarix (GlaxoSmithKline), a vaccine against HPV, which causes cervical cancer. The vaccine is composed of recombinant capsid proteins from types 16 and 18 HPV as virus-like particles, with a combination of aluminium hydroxide and MPL (AS04) as the adjuvant. Cervarix had previously won European Union (EU) approval in 2007, but was the first vaccine licensed by the FDA that includes a TLR agonist as an adjuvant component. A recent study reported a good safety profile for the AS04 adjuvant in more than 68,000 individuals [13].
The oil-in-water emulsion adjuvant MF59 from Novartis (Basel, Switzerland) has been included in an EU-licensed influenza vaccine (Fluad; Novartis) for more than 10 years, has been administered to more than 50 million people, and has accumulated a significant safety database [14]. MF59 was shown to be more potent for both antibody and T-cell responses than aluminum-based adjuvants [14]. Recently, MF59 was shown to be an effective delivery system for a synthetic TLR4 agonist (E6020) in modulating the immune response to a subunit influenza vaccine [15]. Combining adjuvants like E6020 and MF59 allowed selective induction of the immune responses, in particular the induction of helper T (Th) 1 cells, which are important in protection against intracellular pathogens (e.g., influenza vaccines). MF59 activates DC, but its mechanism of action and the possible involvement of PRR are unknown [16]. Mosca et al. [17] demonstrated that MF59 together with CpG and alum activated innate immunity at the site of injection. Using microarray analysis, the early genes induced in gluteus muscle of mice were examined by different adjuvant formulations. The cluster of genes modulated by all adjuvants, named “adjuvant core response genes,” were characterized by the up-regulation of cytokines (IL-1β and IL-2), chemokines (e.g., CCL2, CCL12, and CXCL10), and adhesion molecules, suggesting the establishment of an immunocompetent environment at the site of injection [17].
TLR3
It has been demonstrated that poly I:C, a synthetic analog of double-stranded RNA (dsRNA) and an agonist for TLR3 and MDA5, has mucosal activity when coadministered intranasally with an inactivated influenza vaccine. It enhanced mucosal and systemic humoral responses, resulting in complete protection against homologous and heterologous influenza viruses, including the highly pathogenic H5N1 avian influenza virus [18]. The adjuvant effects of poly I:C require cooperative activation of TLR3 and cytoplasmic RNA helicase MDA5 pathways [19]. PolyI:polyC12U (Ampligen; Hemispherx Biopharma, Philadelphia, PA), which is similar to poly I:C, was reported to have a low incidence of clinical toxicity [20]. More than 75,000 doses of Ampligen have been administered to humans and were generally well tolerated [18].
TLR5
Flagellin, the protein component of bacterial flagella (which aids bacterial mobility), is an agonist for TLR5 and is a potent adjuvant in mice, as well as in cynomolgus and African green monkeys [21–23]. Pseudomonas aeruginosa, a gram-negative bacterium and opportunistic pathogen, is a major cause of morbidity and mortality in cystic fibrosis (CF) patients. A phase 3 clinical trial of P. aeruginosa flagellin in CF patients demonstrated that a vaccine containing flagella subtype antigens a0, a1, a2, and b was well tolerated and caused a 30% reduction in the incidence of infection [24].
TLR7/8
Single-stranded RNA is a ligand for mouse TLR7 and human TLR8 [25]. Synthetic compounds that bind to TLR7/8 induce activation and maturation of DC. The small-molecule nucleotide analogues imiquimod and resiquimod are ligands for TLR7 and TLR7/8, respectively [26]. Several synthetic imidazoquinolines were shown to have potent antiviral and antitumor effects because of their ability to induce inflammatory cytokines, especially IFN-α. One of these imidazoquinoline compounds, imiquimod, has been approved for the treatment of certain cancers and for HPV infection [27].
TLR9
Bacterial DNA, which contains unmethylated CpG dinucleotides, is a ligand for TLR9. Synthetic oligonucleotide (ODNs) containing CpG motifs are potent vaccine adjuvants and their function is mediated through activation of TLR9 signaling pathways [28]. CpG ODNs have shown to be effective adjuvant in clinical trials in humans. However, in 2008, a clinical trial of a hepatitis B candidate vaccine, Heplisav (Dynavax, Berkeley, CA), comprising recombinant hepatitis B surface antigen mixed with a synthetic oligonucleotide containing CpG as the adjuvant, was put on hold by the FDA because of safety concerns. One subject in the trial developed Wegener’s granulomatosis, a severe autoimmune disease in which blood vessels become inflamed. However, a second phase 3 trial is expected to begin in early 2010 for individuals with chronic kidney disease. A recent cancer vaccine clinical trial with CpG-ODN also was abandoned because of unexpectedly weaker responses in humans relative to those observed in mice [29]. This response was attributed to a lower frequency of TLR9 expression on human than mouse immune cells.
TLR Synergy
It has become increasingly evident that molecules that activate the innate immune system through TLRs can promote adaptive immunity to vaccine antigens, but are not always effective on their own. On the other hand, many TLRs were shown to work synergistically. This principle was applied in the development of an experimental vaccine against H1N1 and H5N1 influenza viruses. A combination of MPL and a synthetic TLR7 ligand allowed the dose of H5N1 antigen to be dramatically decreased in mice; as little as 0.1 µg of antigen when combined with the experimental adjuvant had the same effect as a 10-µg antigen dose combined with alum [30].
Modulating TLR Signaling to Improve Adjuvant Activity
Although TLR ligands are capable of promoting Th1 and Th17 responses to coadministered antigens, which are required for protective immunity to many pathogens, they can also induce regulatory T (Treg) cells. This finding reflects the fact that TLR activation of DCs and other innate cells results in the production of anti-inflammatory cytokines (eg, IL-10), which promote Treg cells, as well as proinflammatory cytokines, including IL-1, IL-12, and IL-23, which promote the induction of Th1 and Th17 cells [31]. We have found that IL-10, but not IL-12, production by DCs activated with CpG, LPS, or poly I:C is mediated by activation of p38 mitogen-activated protein kinase [32•]. Inhibition of p38 suppressed CpG-induced IL-10 and enhanced IL-12 production by DCs. Furthermore, addition of a p38 inhibitor to an experimental acellular pertussis vaccine formulated with CpG as the adjuvant suppressed the induction of Treg cells, and enhanced Th1 responses and protection against Bordetella pertussis challenge [32•].
TLR-Independent Adjuvant Activity
Recent studies in mice have shown that, at least for antibody responses, which are the basis of protection induced with most currently licensed vaccines, TLR signaling may not be as important as previously thought. Mice deficient in critical signaling components for TLR (e.g., MyD88 and TRIF) were able to mount robust antibody responses to T cell-dependent antigen when administered with either alum, incomplete or complete Freund’s adjuvant, and MPL [33]. In the absence of TLRs, unconventional adjuvant-containing vehicles (e.g., viruses and apoptotic cells) could elicit efficient adaptive immune responses to a vaccine [34]. Furthermore, Sanders et al. [35] demonstrated that the absence of TLR5 did not have a substantial impact on the ability of flagellin to promote T cell–dependent antibody response to itself or a bystander antigen. Signaling through new families of intracellular PRRs, such as RLRs and NLRs recently were demonstrated to activate innate immune responses and the subsequent adaptive immune response; agonists for these receptors may mediate TLR-independent adjuvant activity. Indeed, the NLR IL-1β-converting enzyme (ICE)-protease-activating factor (IPAF) was shown to recognize flagellin [36]. However, other studies found that TLR activation is essential for the adjuvant activity of certain vaccines. We have demonstrated an absolute requirement for TLR4 in protection induced with the pertussis whole-cell vaccine [3]. The mechanism appears to involve TLR4-mediated activation of DCs by LPS present in the vaccine, which in turn activated Th1 and Th17 cells that conferred protective immunity. It was also demonstrated that protection with the yellow fever vaccine involves activation of plasmacytoid and other DC subtypes through multiple TLRs [4].
RLRs
In addition to TLRs, several distinct cytosolic receptors for RNAs are used by the innate immune system to initiate antiviral responses. These include a family of RNA helicase receptors referred to as RLRs. Members of the RLR family include RIG-1, melanoma differentiation factor 5 (MDA5), and laboratory of genetics and physiology-2 (LGP2) [19]. Unlike TLRs that recognize viral nucleic acids in the endosomes, the RLRs recognize signatures of virus replication within the cytosol of infected cells. Most viruses produce dsRNA in infected cells. Initially, both RIG-1 and melanoma MDA5 were identified as sensors of the synthetic analog of dsRNA, poly I:C. RIG-1 was reported to be involved in the detection of poly I:C, and the subsequent activation of transcription factors nuclear factor κB and IFN regulatory factors 3 and 7, leading to inflammatory cytokine and type 1 IFN production [37]. After recognition of viral RNA, RIG-1 and MDA5 bind to IFN-β promoter stimulator (IPS)-1 via the CARD domain. IPS-1 is localized in the mitochondria and acts as an adaptor that links RLRs to type 1 IFN production [38]. Dissecting the signaling pathways involved in type 1 IFN production has been used to develop a novel adjuvant. Kobiyama et al. [39•] generated several different IPS-1 CARD-fusion polypeptides. Administration of one such polypeptide, N′-CARD polypeptide fused to a protein transduction domain (PTD), elicited production of type I IFNs, maturation of DCs, and enhanced immunogenicity of the antigen by promoting Th1 responses, which conferred protection against lethal influenza infection in mice [39•]. The N′-CARD-PTD polypeptide has the ability to self-transmigrate into the nucleus and trigger activation of innate immune cells in the absence of TLRs but in the presence of NDH and TBK-1, which are ubiquitously expressed in a wide range of cell types. N′-CARD-PTD represents a novel and possible candidate adjuvant in future vaccine development. Modulation of intracellular signaling using cell-permeable polypeptides is a promising technology for future clinical applications. The findings of this study also provide insights that may prove useful in the rational design of immunomodulatory agents, such as the use of constitutively active signaling of the innate immune response [39•].
NLRs
NLRs are a family of cytoplasmic PRRs that contain three distinct domains: C-terminal LRRs, mediating ligand sensing; a centrally located NACHT domain, mediating self-oligomerization and the activation of NLRs; and an N-terminal domain, mediating protein-protein interactions for initiating downstream signaling. NLRs are grouped into several subfamilies on the basis of their effector domains and on the history of their NACHT domains: NODs; NACHT-, Leucine-rich-repeat-, and Pyrin-domain-containing Proteins (NALPs); IPAF; and neuronal apoptosis inhibitory proteins (NAIPs). The NOD and IPAF families contain CARD effector domains, whereas the NALPs and NAIPs contain pyrin (PYD) effector domains and three Baculoviral-Inhibitor of apoptosis protein-Repeats (BIR) domains, respectively. Although the ligands and functions of many of these PRRs are unknown, it appears their major role is to recognize cytoplasmic microbial PAMPs and/or endogenous danger signals, initiating immunologic responses.
Muramyl dipeptide (MDP), the minimal unit of peptidoglycan, was first recognized as the minimum effective component of complete Freund’s adjuvant (CFA) in 1972 [40]. Recently, the adjuvanticity of MDP was shown to be NOD2 dependent, because NOD2-deficient mice are unable to mount a normal humoral response after immunization with MDP with antigen [41]. Interestingly, the adjuvanticity of MDP is altered depending on the formulation used; in saline solution, MDP mainly enhances humoral immunity, but when used in conjunction with lipophilic carriers (eg, liposomes), it induces a strong cellular immune response. However, pyrogenicity problems have restricted the use of MDP for vaccination purposes in humans. In an attempt to circumvent the toxicity problems, several synthetic analogues of MDP were developed, including murabutide and muramyl tripeptide dipalmitoyl phosphatidylethanolamine (MTP-PE). However, in an early clinical testing, MTP-PE proved to be reactogenic and poorly tolerated [42].
Several NLRs (NALPs and IPAF) form a caspase-1–activating multiprotein complex, termed the inflammasome, which processes proinflammatory cytokines, including IL-1β. Among the various inflammasomes, the NALP3 inflammasome is particularly effective at sensing diverse molecules, including bacterial and viral PAMPs, stress-associated danger signals, such as adenosine triphosphate (ATP) or monosodium urate crystals (MSU), as well as asbestos, silica, alum, and β-amyloid [43].
It has been recognized for several years that IL-1β may have an adjuvant capacity; the addition of IL-1 enhanced serum antibody production in mice immunized with protein antigens [44]. Caspase-1, also known as IL-1β–converting enzyme, processes and activates pro-IL-1β and pro-IL-18. Pro-IL-1β lacks a signal peptide and so remains inside the cell. Caspase-1 cleaves pro-IL-1β into active mature IL-1β, which is then secreted into the extracellular space [43]. It is now generally accepted that activation and release of IL-1β requires two distinct signals: the first signal leads to the transcriptional up-regulation and synthesis of pro-IL-1β and other components necessary for inflammasome function (eg, NALP3 itself); the second signal leads to NALP3 inflammasome complex formation, caspase-1 activation, and IL-1β cleavage.
Recent reports indicate that virus infection also results in the activation of the inflammasome. Both Sendai and influenza viruses activated NALP3 inflammasome in macrophages pulsed transiently with ATP for 30 min in vitro [45]. Uric acid crystals activate NALP3 inflammasome [46], and it was reported that influenza virus can induce uric acid in bronchoalveolar lavage fluid and serum in mice [47]. Shi et al. [48] demonstrated that uric acid that is released from injured cells stimulated DC maturation, and when coinjected with HIV-1 gp120 antigen in vivo, significantly enhanced CD8 T-cell responses.
Insoluble ammonium salts (alum) is the most widely used adjuvant in human vaccines, and has been included in licensed vaccines worldwide for more than 50 years. Although the mechanism of action of alum is unclear, reports implicated the NALP3 inflammasome in its immunostimulatory activity [49, 50•, 51, 52]. The adjuvant action of alum was shown to require NALP3 activation in a mouse model of allergic airway disease in which alum was administered intraperitoneally with ovalbumin [50•]. Mice deficient in NALP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), or caspase-1 failed to mount a significant antibody response to an antigen administered with aluminium adjuvants, but had intact responses with CFA [50•]. NALP3 was shown to be required for the generation of antigen-specific antibody responses to antigen administered with alum as the adjuvant [51, 52]. Interestingly, Li et al. [52] demonstrated that the adjuvants QuilA and chitosan also activated the NALP3 inflammasome in vitro, suggesting that particulate adjuvants may share a common mechanism of action. Furthermore, the enhancement of IL-1β production by DC through NALP3 appears to be a general property shared by particulate adjuvants [53•]. The generation of potent antigen-specific antibody response with biodegradable poly (lactide-co-glycolide) (PLG) microparticles was not dependent on NALP3. However, NALP3-deficient mice had defective antigen-specific cell-mediated immunity when PLG was used as an adjuvant [53•].
C-type Lectin Receptors
C-type lectin receptors (CLRs) encompass a large family of proteins with varied functions. They contain one or more C-type lectin domains, which mediate carbohydrate binding in a Ca2+ dependent manner [54]. CFA, an emulsion, incorporating killed Mycobacterium tuberculosis, efficiently induces Th1 responses in mice, but is too toxic for use in humans. In the search for alternative adjuvants based on CFA that are both safe and effective, purified PAMPs and their synthetic analogues were investigated. CLRs were implicated in the recognition of certain mycobacterial cell wall components [55]. One such component, trehalose-6,6-dimycolate (TDM) has potent inflammatory activity [56], and when used alone or in combination with a TLR4 ligand has adjuvant activity in mice [57, 58]. The less toxic analogue trehalose-6,6-dibehenate (TDB) induced robust Th1 responses after immunization with the recombinant M. tuberculosis antigen H1, conferring protection against challenge and reducing the bacterial load as effectively as the traditional bacille Calmette-Guérin vaccine [59, 60]. Both the mycobacteria-derived glycolipid TDM and the synthetic adjuvant TDB selectively activate the FcRγ-Syk-Card9 pathway in APC to induce a unique innate immune activation program that directs protective Th1 and Th17 cells [55]. Interestingly, the binding of the β-glucans curdlan or zymosan to the CLR dectin-1 activates the Syk kinase, initiating signaling via the Card9-Bcl10-Malt1 pathway, and directs Th17 cell differentiation [61]. A recent study, however, ruled out dectin-1 as the receptor responsible for TDB and TDM recognition, but identified other possible CLR candidates, including DC associated C-type lectin 2 (dectin-2) and DC immunostimulatory receptor (DCAR) [55].
Crosstalk Between PRRs
Evaluating combinations of PAMPs may be a useful strategy in future adjuvant design. Multicomponent adjuvants could potentiate an immune response and/or skew the immune response appropriately depending on the infectious disease target. Indeed, examples of synergy and crosstalk between TLR agonists and between TLR and NOD agonists have already been reported. TLR3 and TLR4 agonists strongly synergize with agonists of TLR7, 8, and 9, leading to a Th1-polarized response [62]. TLR4 agonists were also shown to synergize with NOD1 and NOD2 agonists to induce DC maturation [63].
Conclusions
Vaccines are crucial for the prevention of infectious diseases. A better understanding of the biologic basis of existing vaccines may provide clues for optimal development strategies for future adjuvants and vaccines. It may also provide explanations for past vaccination failures. Collectively, such information should pave the way toward a new generation of highly immunogenic, low-risk vaccines. Although concerns have been raised about the potential safety of PRR agonists as adjuvants in new-generation human vaccines, many current vaccines are effective because they include a range of PAMPs. For example, whole bacterial vaccines (eg, the whole-cell pertussis vaccine) contain significant amounts of LPS and bacterial DNA [3]. A better understanding of the mechanisms of action of adjuvants should help in generating more effective and safer vaccines against infectious diseases.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Janeway CA Jr: Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 1989, 54(Pt 1):1-13.
Janeway CA Jr, Medzhitov R: Innate immune recognition. Annu Rev Immunol 2002, 20:197–216.
Higgins SC, Jarnicki AG, Lavelle EC, Mills KH: TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17–producing T cells. J Immunol 2006, 177:7980–7989.
Querec T, Bennouna S, Alkan S, et al.: Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 2006, 203:413–424.
Medzhitov R: Toll-like receptors and innate immunity. Nat Rev Immunol 2001, 1:135–145.
Condie RM, Zak SJ, Good RA: Effect of meningococcal endotoxin on the immune response. Proc Soc Exp Biol Med 1955, 90:355–360.
Baldridge JR, Crane RT: Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods 1999, 19:103–107.
Blander JM, Medzhitov R: Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 2006, 440:808–812.
Bojang KA, Olodude F, Pinder M, et al.: Safety and immunogenicty of RTS,S/AS02A candidate malaria vaccine in Gambian children. Vaccine 2005, 23:4148–4157.
Bovier PA, Farinelli T, Loutan L: Interchangeability and tolerability of a virosomal and an aluminum-adsorbed hepatitis A vaccine. Vaccine 2005, 23:2424–2429.
Kaufmann SH: Tuberculosis and AIDS—a devilish liaison. Drug Discov Today 2007, 12(21–22):891–893.
McCormack S, Tilzey A, Carmichael A, et al.: A phase I trial in HIV negative healthy volunteers evaluating the effect of potent adjuvants on immunogenicity of a recombinant gp120W61D derived from dual tropic R5X4 HIV-1ACH320. Vaccine 2000, 18:1166–1177.
Verstraeten T, Descamps D, David MP, et al.: Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008, 26:6630–6638.
O’Hagan DT, De Gregorio E: The path to a successful vaccine adjuvant—‘the long and winding road.’ Drug Discov Today 2009, 14(11–12):541–551.
Baudner BC, Ronconi V, Casini D, et al.: MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020). Pharm Res 2009, 26:1477–1485.
Dupuis M, Murphy TJ, Higgins D, et al.: Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol 1998, 186:18–27.
Mosca F, Tritto E, Muzzi A, et al.: Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A 2008, 105:10501–10506.
Ichinohe T, Tamura S, Kawaguchi A, et al.: Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J Infect Dis 2007, 196:1313–1320.
Kumar H, Koyama S, Ishii KJ, et al.: Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol 2008, 180:683–687.
Suhadolnik RJ, Reichenbach NL, Hitzges P, et al.: Upregulation of the 2-5A synthetase/RNase L antiviral pathway associated with chronic fatigue syndrome. Clin Infect Dis 1994, 18(Suppl 1):S96–S104.
Bates JT, Honko AN, Graff AH, et al.: Mucosal adjuvant activity of flagellin in aged mice. Mech Ageing Dev 2008, 129:271–281.
Honko AN, Mizel SB: Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun 2004, 72:6676–6679.
McSorley SJ, Ehst BD, Yu Y, Gewirtz AT: Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol 2002, 169:3914–3919.
Doring G, Meisner C, Stern M: A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc Natl Acad Sci U S A 2007, 104:11020–11025.
Heil F, Hemmi H, Hochrein H, et al.: Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303:1526–1529.
Gorden KB, Gorski KS, Gibson SJ, et al.: Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 2005, 174:1259–1268.
Hemmi H, Kaisho T, Takeuchi O, et al.: Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 2002, 3:196–200.
Klinman DM, Xie H, Little SF, et al.: CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine 2004, 22(21–22):2881–2886.
Schmidt C: Clinical setbacks for toll-like receptor 9 agonists in cancer. Nat Biotechnol 2007, 25:825–826.
Schubert C: Boosting our best shot. Nat Med 2009, 15:984–988.
Conroy H, Marshall NA, Mills KH: TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene 2008, 27:168–180.
• Jarnicki AG, Conroy H, Brereton C, et al.: Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol 2008, 180:3797–3806. This article illustrates the limitations of TLR agonists as adjuvants and how they can be made more effective by modulating downstream signaling pathways.
Gavin AL, Hoebe K, Duong B, et al.: Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 2006, 314:1936–1938.
Lopez CB, Moltedo B, Alexopoulou L, et al.: TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J Immunol 2004, 173:6882–6889.
Sanders CJ, Franchi L, Yarovinsky F, et al.: Induction of adaptive immunity by flagellin does not require robust activation of innate immunity. Eur J Immunol 2009, 39:359–371.
Miao EA, Alpuche-Aranda CM, Dors M, et al.: Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 2006, 7:569–575.
Kato H, Sato S, Yoneyama M, et al.: Cell type-specific involvement of RIG-I in antiviral response. Immunity 2005, 23:19–28.
Kawai T, Takahashi K, Sato S, et al.: IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 2005, 6:981–988.
• Kobiyama K, Takeshita F, Ishii KJ, et al.: A signaling polypeptide derived from an innate immune adaptor molecule can be harnessed as a new class of vaccine adjuvant. J Immunol 2009, 182:1593–1601. These authors showed that a polypeptide corresponding to an intracellular signaling molecule in the RIG-1 pathway could enhanced immune responses in vivo and therefore had potential as an adjuvant.
Adam A, Ciorbaru R, Petit JF, Lederer E: Isolation and properties of a macromolecular, water-soluble, immuno-adjuvant fraction from the cell wall of Mycobacterium smegmatis. Proc Natl Acad Sci U S A 1972, 69:851–854.
Magalhaes JG, Fritz JH, Le Bourhis L, et al.: Nod2-dependent Th2 polarization of antigen-specific immunity. J Immunol 2008, 181:7925–7935.
Keitel W, Couch R, Bond N, et al.: Pilot evaluation of influenza virus vaccine (IVV) combined with adjuvant. Vaccine 1993, 11:909–913.
Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G: The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009, 10:241–247.
Staruch MJ, Wood DD: The adjuvanticity of interleukin 1 in vivo. J Immunol 1983, 130:2191–2194.
Kanneganti TD, Body-Malapel M, Amer A, et al.: Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 2006, 281:36560–36568.
Martinon F, Tschopp J: Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 2007, 14:10–22.
Akaike T, Ando M, Oda T, et al.: Dependence on O2-generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest 1990, 85:739–745.
Shi Y, Evans JE, Rock KL: Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003, 425:516–521.
Hornung V, Bauernfeind F, Halle A, et al.: Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008, 9:847–856.
• Eisenbarth SC, Colegio OR, O’Connor W, et al.: Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008, 453:1122–1126. This article provided the first evidence that the mechanism of action of alum may be mediated through activation of innate immunity (Editor: make this two star reference).
Kool M, Petrilli V, De Smedt T, et al.: Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol 2008, 181:3755–3759.
Li H, Willingham SB, Ting JP, Re F: Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol 2008, 181:17–21.
• Sharp FA, Ruane D, Claass B, et al.: Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A 2009, 106:870–875. This article shows that biodegradable microparticles have adjuvant as well as vaccine delivery properties, and that this activity is mediated through activation of the inflammasome.
Geijtenbeek TB, Gringhuis SI: Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 2009, 9:465–479.
Werninghaus K, Babiak A, Gross O, et al.: Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med 2009, 206:89–97.
Geisel RE, Sakamoto K, Russell DG, Rhoades ER: In vivo activity of released cell wall lipids of Mycobacterium bovis bacillus Calmette-Guerin is due principally to trehalose mycolates. J Immunol 2005, 174, 5007–5015.
Khader SA, Bell GK, Pearl JE, et al.: IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007, 8:369–377.
Lima KM, Santos SA, Lima VM, et al.: Single dose of a vaccine based on DNA encoding mycobacterial hsp65 protein plus TDM-loaded PLGA microspheres protects mice against a virulent strain of Mycobacterium tuberculosis. Gene Ther 2003, 10:678–685.
Davidsen J, Rosenkrands I, Christensen D, et al.: Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta 2005, 1718(1–2):22–31.
Holten-Andersen L, Doherty TM, Korsholm KS, Andersen P: Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect Immun 2004, 72:1608–1617.
LeibundGut-Landmann S, Gross O, Robinson MJ, et al.: Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 2007, 8:630–638.
Napolitani G, Rinaldi A, Bertoni F, et al.: Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol 2005, 6:769–776.
Fritz JH, Girardin SE, Fitting C, et al.: Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol 2005, 35:2459–2470.
Acknowledgements
KM and SH are supported by research grants from Science Foundation Ireland and the EU framework 7th Framework Programme - project NASPANVAC.
Disclosure
Kingston Mills is a co-founder, minority shareholder, and consultant to Opsona Therapeutics Ltd. (Dublin, Ireland). No other potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higgins, S.C., Mills, K.H.G. TLR, NLR Agonists, and Other Immune Modulators as Infectious Disease Vaccine Adjuvants. Curr Infect Dis Rep 12, 4–12 (2010). https://doi.org/10.1007/s11908-009-0080-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-009-0080-9