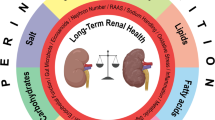
Abstract
Purpose of Review
This review will provide an in-depth coverage of the epidemiological and pre-clinical literature surrounding the role of dietary protein in hypertension, with a special emphasis on the history of our work on the Dahl salt-sensitive rat.
Recent Findings
Our studies have dedicated much effort into understanding the relationship between dietary protein and its effect on the development of salt-sensitive hypertension and renal injury. Our evidence over the last 15 years have demonstrated that both the source and amount of dietary protein can influence the severity of disease, where we have determined mechanisms related to immunity, the maternal environment during pregnancy, and more recently the gut microbiota, which significantly contribute to these diet-induced effects.
Summary
Deeper understanding of these dietary protein-related mechanisms may provide insight on the plausibility of dietary modifications as future therapeutic avenues for hypertension and renal disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. https://doi.org/10.1161/HYP.0000000000000065.
Hajjar I, Kotchen T. Regional variations of blood pressure in the United States are associated with regional variations in dietary intakes: the NHANES-III data. J Nutr. 2003;133(1):211–4. https://doi.org/10.1093/jn/133.1.211.
Kesteloot H, Joossens JV. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension. 1988;12(6):589–93. https://doi.org/10.1161/01.hyp.12.6.589.
Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA. Relationship to blood pressure of combinations of dietary macronutrients. Findings of the Multiple Risk Factor Intervention Trial (MRFIT). Circulation. 1996;94(10):2417–23. https://doi.org/10.1161/01.cir.94.10.2417.
Vasdev S, Stuckless J. Antihypertensive effects of dietary protein and its mechanism. Int J Angiol. 2010;19(1):e7–e20.
Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med. 2001;161(4):589–93. https://doi.org/10.1001/archinte.161.4.589.
Malhotra R, Lipworth L, Cavanaugh KL, Young BA, Tucker KL, Carithers TC, et al. Protein intake and long-term change in glomerular filtration rate in the Jackson Heart Study. J Ren Nutr. 2018;28(4):245–50. https://doi.org/10.1053/j.jrn.2017.11.008.
Watanabe D, Machida S, Matsumoto N, Shibagaki Y, Sakurada T. Age modifies the association of dietary protein intake with all-cause mortality in patients with chronic kidney disease. Nutrients. 2018;10(11). https://doi.org/10.3390/nu10111744.
Lentine K, Wrone EM. New insights into protein intake and progression of renal disease. Curr Opin Nephrol Hypertens. 2004;13(3):333–6. https://doi.org/10.1097/00041552-200405000-00011.
Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev. 2009;3:CD001892. https://doi.org/10.1002/14651858.CD001892.pub3.
Sacks FM, Rosner B, Kass EH. Blood pressure in vegetarians. Am J Epidemiol. 1974;100(5):390–8. https://doi.org/10.1093/oxfordjournals.aje.a112050.
Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, et al. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med. 2006;166(1):79–87. https://doi.org/10.1001/archinte.166.1.79.
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. https://doi.org/10.1056/NEJM199704173361601.
Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–64. https://doi.org/10.1001/jama.294.19.2455.
Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk? Adv Nutr. 2015;6(6):712–28. https://doi.org/10.3945/an.115.009654.
Sun D, Zhou T, Li X, Heianza Y, Liang Z, Bray GA, et al. Genetic susceptibility, dietary protein intake, and changes of blood pressure: the pounds lost trial. Hypertension. 2019. https://doi.org/10.1161/HYPERTENSIONAHA.119.13510.
Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Effect of high fat loading in Dahl salt-sensitive rats. Clin Exp Hypertens. 2009;31(5):451–61.
Shimokawa T, Moriuchi A, Hori T, Saito M, Naito Y, Kabasawa H, et al. Effect of dietary alpha-linolenate/linoleate balance on mean survival time, incidence of stroke and blood pressure of spontaneously hypertensive rats. Life Sci. 1988;43(25):2067–75. https://doi.org/10.1016/0024-3205(88)90356-6.
Spradley FT, De Miguel C, Hobbs J, Pollock DM, Pollock JS. Mycophenolate mofetil prevents high-fat diet-induced hypertension and renal glomerular injury in Dahl SS rats. Phys Rep. 2013;1(6):e00137. https://doi.org/10.1002/phy2.137.
Zhang HY, Reddy S, Kotchen TA. A high sucrose, high linoleic acid diet potentiates hypertension in the Dahl salt sensitive rat. Am J Hypertens. 1999;12(2 Pt 1):183–7. https://doi.org/10.1016/s0895-7061(98)00238-6.
Preuss HG, Knapka JJ, MacArthy P, Yousufi AK, Sabnis SG, Antonovych TT. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. Am J Hypertens. 1992;5(9):585–91. https://doi.org/10.1093/ajh/5.9.585.
Young JB, Landsberg L. Effect of oral sucrose on blood pressure in the spontaneously hypertensive rat. Metabolism. 1981;30(5):421–4. https://doi.org/10.1016/0026-0495(81)90173-6.
Nevala R, Vaskonen T, Vehniainen J, Korpela R, Vapaatalo H. Soy based diet attenuates the development of hypertension when compared to casein based diet in spontaneously hypertensive rat. Life Sci. 2000;66(2):115–24. https://doi.org/10.1016/s0024-3205(99)00569-x.
Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension. 1994;23(2):195–9. https://doi.org/10.1161/01.hyp.23.2.195.
Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, et al. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics. 2004;16(2):194–203. https://doi.org/10.1152/physiolgenomics.00151.2003.
Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2005;45(4):736–41. https://doi.org/10.1161/01.HYP.0000153318.74544.cc.
De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension. 2011;57(2):269–74. https://doi.org/10.1161/HYPERTENSIONAHA.110.154302.
Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Mattson DL. Rag1-null Dahl SS rats reveal that adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am J Phys Regul Integr Comp Phys. 2018;315(1):R28–35. https://doi.org/10.1152/ajpregu.00201.2017.
Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Alsheikh AJ, Mattson DL. Parental dietary protein source and the role of CMKLR1 in determining the severity of Dahl salt-sensitive hypertension. Hypertension. 2019;73(2):440–8. https://doi.org/10.1161/HYPERTENSIONAHA.118.11994.
Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, et al. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 2015;65(2):447–55. https://doi.org/10.1161/HYPERTENSIONAHA.114.04179.
Abais-Battad JM, Alsheikh AJ, Pan X, Fehrenbach DJ, Dasinger JH, Lund H, et al. Dietary effects on Dahl salt-sensitive hypertension, renal damage, and the T lymphocyte transcriptome. Hypertension. 2019;74(4):854–63. https://doi.org/10.1161/HYPERTENSIONAHA.119.12927.
Dasinger JH, Alsheikh AJ, Abais-Battad JM, Pan X, Fehrenbach DJ, Lund H, et al. Epigenetic modifications in T cells: the role of DNA methylation in salt-sensitive hypertension. Hypertension. 2019. https://doi.org/10.1161/HYPERTENSIONAHA.119.13716.
Lopez-Legarrea P, de la Iglesia R, Abete I, Navas-Carretero S, Martinez JA, Zulet MA. The protein type within a hypocaloric diet affects obesity-related inflammation: the RESMENA project. Nutrition. 2014;30(4):424–9. https://doi.org/10.1016/j.nut.2013.09.009.
Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: the E3N prospective study. Am J Gastroenterol. 2010;105(10):2195–201. https://doi.org/10.1038/ajg.2010.192.
Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53(10):1479–84. https://doi.org/10.1136/gut.2003.024828.
Mohammad A, Ota F, Kassu A, Sorayya K, Sakai T. Modulation of oral tolerance to ovalbumin by dietary protein in mice. J Nutr Sci Vitaminol (Tokyo). 2006;52(2):113–20. https://doi.org/10.3177/jnsv.52.113.
• Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351(6275):858–63. https://doi.org/10.1126/science.aac5560This study demonstrated that dietary antigens, in the form of whole proteins, are necessary for proper production of intestinal Tregs.
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. https://doi.org/10.1073/pnas.1005963107.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. https://doi.org/10.1038/nature12820.
Macfarlane GT, Cummings JH, Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986;132(6):1647–56. https://doi.org/10.1099/00221287-132-6-1647.
Dai ZL, Zhang J, Wu G, Zhu WY. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. 2010;39(5):1201–15. https://doi.org/10.1007/s00726-010-0556-9.
Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, et al. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res. 2013;68(1):95–107. https://doi.org/10.1016/j.phrs.2012.11.005.
• Diether NE, Willing BP. Microbial fermentation of dietary protein: an important factor in diet(-)microbe(-)host interaction. Microorganisms. 2019;7(1). https://doi.org/10.3390/microorganisms7010019In-depth review on the reciprocal relationship between gut microbiota and proteolytic fermentation byproducts.
Wen S, Zhou G, Song S, Xu X, Voglmeir J, Liu L, et al. Discrimination of in vitro and in vivo digestion products of meat proteins from pork, beef, chicken, and fish. Proteomics. 2015;15(21):3688–98. https://doi.org/10.1002/pmic.201500179.
Kar SK, Jansman AJM, Benis N, Ramiro-Garcia J, Schokker D, Kruijt L, et al. Dietary protein sources differentially affect microbiota, mTOR activity and transcription of mTOR signaling pathways in the small intestine. PLoS One. 2017;12(11):e0188282. https://doi.org/10.1371/journal.pone.0188282.
Zhu Y, Shi X, Lin X, Ye K, Xu X, Li C, et al. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front Microbiol. 2017;8:1395. https://doi.org/10.3389/fmicb.2017.01395.
• Kim E, Kim DB, Park JY. Changes of mouse gut microbiota diversity and composition by modulating dietary protein and carbohydrate contents: a pilot study. Prev Nutr Food Sci. 2016;21(1):57–61. https://doi.org/10.3746/pnf.2016.21.1.57This study provides clear evidence for the adverse effects of a high casein diet on the microbiota composition and intestinal health of mice.
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40. https://doi.org/10.1161/HYPERTENSIONAHA.115.05315.
Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47(6):187–97. https://doi.org/10.1152/physiolgenomics.00136.2014.
Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96–104. https://doi.org/10.1152/physiolgenomics.00081.2016.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551(7682):585–9. https://doi.org/10.1038/nature24628.
•• Ferguson JF, Aden LA, Barbaro NR, Van Beusecum JP, Xiao L, Simmons AJ, et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight. 2019;5. https://doi.org/10.1172/jci.insight.126241Recent publication demonstrating the interplay between increased salt consumption, antigen presenting cell activation, and changes to the gut microbiota.
Abais-Battad JM, Saravia FL, Lund H, Dasinger JH, Fehrenbach DJ, Alsheikh AJ, et al. Role of gut microbiota and immunity in the dietary modulation of Dahl salt-sensitive hypertension. FASEB J. 2019;33:866.9.
Kenny LC, Black MA, Poston L, Taylor R, Myers JE, Baker PN, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension. 2014;64(3):644–52. https://doi.org/10.1161/HYPERTENSIONAHA.114.03578.
Brantsaeter AL, Haugen M, Samuelsen SO, Torjusen H, Trogstad L, Alexander J, et al. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr. 2009;139(6):1162–8. https://doi.org/10.3945/jn.109.104968.
Qiu C, Coughlin KB, Frederick IO, Sorensen TK, Williams MA. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am J Hypertens. 2008;21(8):903–9. https://doi.org/10.1038/ajh.2008.209.
Dasinger JH, Abais-Battad JM, Fehrenbach DJ, Lund H, Zemaj J, Alsheikh AJ, et al. Downregulation of placental genes are associated with the development of maternal syndrome in Dahl salt-sensitive rats. Hypertension. 2019;74:A152.
Liu J, Yang H, Yin Z, Jiang X, Zhong H, Qiu D, et al. Remodeling of the gut microbiota and structural shifts in preeclampsia patients in South China. Eur J Clin Microbiol Infect Dis. 2017;36(4):713–9. https://doi.org/10.1007/s10096-016-2853-z.
Lv LJ, Li SH, Li SC, Zhong ZC, Duan HL, Tian C, et al. Early-onset preeclampsia is associated with gut microbial alterations in antepartum and postpartum women. Front Cell Infect Microbiol. 2019;9:224. https://doi.org/10.3389/fcimb.2019.00224.
Nordqvist M, Jacobsson B, Brantsaeter AL, Myhre R, Nilsson S, Sengpiel V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: a prospective observational cohort study in Norway. BMJ Open. 2018;8(1):e018021. https://doi.org/10.1136/bmjopen-2017-018021.
• Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–80. https://doi.org/10.1016/j.cell.2012.07.008This study highlights the vast microbiotal changes that occur throughout pregnancy and impacts host metabolism, inflammation, and adiposity.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. https://doi.org/10.1073/pnas.1002601107.
Warren MF, Hallowell HA, Higgins KV, Liles MR, Hood WR. Maternal dietary protein intake influences milk and offspring gut microbial diversity in a rat (Rattus norvegicus) model. Nutrients. 2019;11(9). https://doi.org/10.3390/nu11092257.
Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, et al. Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PLoS One. 2016;11(4):e0152751. https://doi.org/10.1371/journal.pone.0152751.
Salazar Garcia MD, Mobley Y, Henson J, Davies M, Skariah A, Dambaeva S, et al. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J Reprod Immunol. 2018;125:25–31. https://doi.org/10.1016/j.jri.2017.10.048.
Darmochwal-Kolarz D, Saito S, Rolinski J, Tabarkiewicz J, Kolarz B, Leszczynska-Gorzelak B, et al. Activated T lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2007;58(1):39–45. https://doi.org/10.1111/j.1600-0897.2007.00489.x.
Jafri S, Ormiston ML. Immune regulation of systemic hypertension, pulmonary arterial hypertension, and preeclampsia: shared disease mechanisms and translational opportunities. Am J Phys Regul Integr Comp Phys. 2017;313(6):R693–705. https://doi.org/10.1152/ajpregu.00259.2017.
Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J, et al. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am J Phys Regul Integr Comp Phys. 2016;311(6):R1192–R9. https://doi.org/10.1152/ajpregu.00117.2016.
Dasinger JH, Abais-Battad JM, Lund H, Fehrenbach DJ, Alsheikh AJ, Mattson DL. T lymphocytes contribute to the development of maternal syndrome in Dahl SS rats maintained on a low salt diet. FASEB J. 2018;32:911.2.
Acknowledgments
We kindly thank Dr. David Mattson for his continued support and guidance. We also acknowledge funding sources: 19CDA34660184.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Inflammation and Cardiovascular Diseases
Rights and permissions
About this article
Cite this article
Dasinger, J.H., Fehrenbach, D.J. & Abais-Battad, J.M. Dietary Protein: Mechanisms Influencing Hypertension and Renal Disease. Curr Hypertens Rep 22, 13 (2020). https://doi.org/10.1007/s11906-020-1018-8
Published:
DOI: https://doi.org/10.1007/s11906-020-1018-8