Abstract
Purpose of Review
This article provides a concise overview of how cannabinoids and the endocannabinoid system (ECS) have significant implications for the prevention and treatment of metabolic syndrome (MetS) and for the treatment of cardiovascular disorders, including cardiac fibrosis.
Recent Findings
Over the past few years, the ECS has emerged as a pivotal component of the homeostatic mechanisms for the regulation of many bodily functions, including inflammation, digestion, and energy metabolism. Therefore, the pharmacological modulation of the ECS by cannabinoids represents a novel strategy for the management of many diseases. Specifically, increasing evidence from preclinical research studies has opened new avenues for the development of cannabinoid-based therapies for the management and potential treatment of MetS and cardiovascular diseases.
Summary
Current information indicates that modulation of the ECS can help maintain overall health and well-being due to its homeostatic function. From a therapeutic perspective, cannabinoids and the ECS have also been shown to play a key role in modulating pathophysiological states such as inflammatory, neurodegenerative, gastrointestinal, metabolic, and cardiovascular diseases, as well as cancer and pain. Thus, targeting and modulating the ECS with cannabinoids or cannabinoid derivatives may represent a major disease-modifying medical advancement to achieve successful treatment for MetS and certain cardiovascular diseases.

Similar content being viewed by others
References
Simon V, Cota D. Mechanisms in endocrinology: endocannabinoids and metabolism: past, present and future. Eur J Endocrinol. 2017;176(6):R309–R24. https://doi.org/10.1530/EJE-16-1044.
Alfulaij N, Meiners F, Michalek J, Small-Howard AL, Turner HC, Stokes AJ. Cannabinoids, the heart of the matter. J Am Heart Assoc. 2018;7(14). https://doi.org/10.1161/JAHA.118.009099.
Mechoulam R, Shvo Y. Hashish. I. The structure of cannabidiol. Tetrahedron. 1963;19(12):2073–8. https://doi.org/10.1016/0040-4020(63)85022-x.
Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93(1):217–24. https://doi.org/10.1021/ja00730a036.
Mechoulam R, Gaoni Y. The absolute configuration of delta-1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 1967;12:1109–11. https://doi.org/10.1016/s0040-4039(00)90646-4.
Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;6869:619–31. https://doi.org/10.1016/s0090-6980(02)00060-6.
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4. https://doi.org/10.1038/346561a0.
Brown SM, Wager-Miller J, Mackie K. Cloning and molecular characterization of the rat CB2 cannabinoid receptor. Biochim Biophys Acta. 2002;1576(3):255–64. https://doi.org/10.1016/s0167-4781(02)00341-x.
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–9. https://doi.org/10.1126/science.1470919.
Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–8. https://doi.org/10.1038/42015.
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–7. https://doi.org/10.1038/384083a0.
Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121(1–2):149–58. https://doi.org/10.1016/s0009-3084(02)00150-0.
Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov. 2018;17(9):623–39. https://doi.org/10.1038/nrd.2018.115.
Joshi N, Onaivi ES. Endocannabinoid system components: overview and tissue distribution. Adv Exp Med Biol. 2019;1162:1–12. https://doi.org/10.1007/978-3-030-21737-2_1.
Di Marzo V, Silvestri C. Lifestyle and metabolic syndrome: contribution of the endocannabinoidome. Nutrients. 2019;11(8). https://doi.org/10.3390/nu11081956.
Rossi F, Punzo F, Umano GR, Argenziano M, Miraglia Del Giudice E. Role of cannabinoids in obesity. Int J Mol Sci. 2018;19(9). https://doi.org/10.3390/ijms19092690.
Ruiz de Azua I, Lutz B. Multiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissues. Cell Mol Life Sci. 2019;76(7):1341–63. https://doi.org/10.1007/s00018-018-2994-6.
Ho WSV, Kelly MEM. Cannabinoids in the cardiovascular system. Adv Pharmacol. 2017;80:329–66. https://doi.org/10.1016/bs.apha.2017.05.002.
Pacher P, Steffens S, Hasko G, Schindler TH, Kunos G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol. 2018;15(3):151–66. https://doi.org/10.1038/nrcardio.2017.130.
Ruiz de Azua I, Mancini G, Srivastava RK, Rey AA, Cardinal P, Tedesco L, et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J Clin Invest. 2017;127(11):4148–62. https://doi.org/10.1172/JCI83626.
Chang E, Kim DH, Yang H, Lee DH, Bae SH, Park CY. CB1 receptor blockade ameliorates hepatic fat infiltration and inflammation and increases Nrf2-AMPK pathway in a rat model of severely uncontrolled diabetes. PLoS One. 2018;13(10):e0206152. https://doi.org/10.1371/journal.pone.0206152.
Bazwinsky-Wutschke I, Zipprich A, Dehghani F. Endocannabinoid system in hepatic glucose metabolism, fatty liver disease, and cirrhosis. Int J Mol Sci. 2019;20(10). https://doi.org/10.3390/ijms20102516.
Nagappan A, Shin J, Jung MH. Role of cannabinoid receptor type 1 in insulin resistance and its biological implications. Int J Mol Sci. 2019;20(9). https://doi.org/10.3390/ijms20092109.
Nava-Molina L, Uchida-Fuentes T, Ramos-Tovar H, Fregoso-Padilla M, Rodriguez-Monroy MA, Vega AV, et al. Novel CB1 receptor antagonist BAR-1 modifies pancreaticislet function and clinical parameters in prediabetic and diabetic mice. Nutr Diabetes. 2020;10(1):7. https://doi.org/10.1038/s41387-020-0110-0.
Jourdan T, Nicoloro SM, Zhou Z, Shen Y, Liu J, Coffey NJ, et al. Decreasing CB1 receptor signaling in Kupffer cells improves insulin sensitivity in obese mice. Mol Metab. 2017;6(11):1517–28. https://doi.org/10.1016/j.molmet.2017.08.011.
Lau BK, Cota D, Cristino L, Borgland SL. Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology. 2017;124:38–51. https://doi.org/10.1016/j.neuropharm.2017.05.033.
Gil-Ordonez A, Martin-Fontecha M, Ortega-Gutierrez S, Lopez-Rodriguez ML. Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem Pharmacol. 2018;157:18–32. https://doi.org/10.1016/j.bcp.2018.07.036.
Grabner GF, Zimmermann R, Schicho R, Taschler U. Monoglyceride lipase as a drug target: at the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther. 2017;175:35–46. https://doi.org/10.1016/j.pharmthera.2017.02.033.
Habib A, Chokr D, Wan J, Hegde P, Mabire M, Siebert M, et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut. 2018;68:522–32. https://doi.org/10.1136/gutjnl-2018-316137.
Shrestha N, Cuffe JSM, Hutchinson DS, Headrick JP, Perkins AV, McAinch AJ, et al. Peripheral modulation of the endocannabinoid system in metabolic disease. Drug Discov Today. 2018;23(3):592–604. https://doi.org/10.1016/j.drudis.2018.01.029.
Rossi F, Bellini G, Luongo L, Manzo I, Tolone S, Tortora C, et al. Cannabinoid receptor 2 as antiobesity target: inflammation, fat storage, and browning modulation. J Clin Endocrinol Metab. 2016;101(9):3469–78. https://doi.org/10.1210/jc.2015-4381.
Puhl SL. Cannabinoid-sensitive receptors in cardiac physiology and ischaemia. Biochim Biophys Acta Mol Cell Res. 1867;2020(3):118462. https://doi.org/10.1016/j.bbamcr.2019.03.009.
Steffens S, Pacher P. Targeting cannabinoid receptor CB(2) in cardiovascular disorders: promises and controversies. Br J Pharmacol. 2012;167(2):313–23. https://doi.org/10.1111/j.1476-5381.2012.02042.x.
del Rio C, Navarrete C, Collado JA, Bellido ML, Gomez-Canas M, Pazos MR, et al. The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-gamma and CB2 pathways. Sci Rep. 2016;6:21703. https://doi.org/10.1038/srep21703.
Li X, Han D, Tian Z, Gao B, Fan M, Li C, et al. Activation of cannabinoid receptor type II by AM1241 ameliorates myocardial fibrosis via Nrf2-mediated inhibition of TGF-beta1/Smad3 pathway in myocardial infarction mice. Cell Physiol Biochem. 2016;39(4):1521–36. https://doi.org/10.1159/000447855.
Horckmans M, Bianchini M, Santovito D, Megens RTA, Springael JY, Negri I, et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis, and cardiac function after myocardial infarction. Circulation. 2018;137(9):948–60. https://doi.org/10.1161/CIRCULATIONAHA.117.028833.
Toczek M, Malinowska B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018;204:20–45. https://doi.org/10.1016/j.lfs.2018.04.054.
Harasim-Symbor E, Polak-Iwaniuk A, Konstantynowicz-Nowicka K, Bielawiec P, Malinowska B, Kasacka I et al. Experimental activation of endocannabinoid system reveals antilipotoxic effects on cardiac myocytes. Molecules. 2020;25(8). https://doi.org/10.3390/molecules25081932.
Pedzinska-Betiuk A, Weresa J, Toczek M, Baranowska-Kuczko M, Kasacka I, Harasim-Symbor E, et al. Chronic inhibition of fatty acid amide hydrolase by URB597 produces differential effects on cardiac performance in normotensive and hypertensive rats. Br J Pharmacol. 2017;174(13):2114–29. https://doi.org/10.1111/bph.13830.
Harasim-Symbor E, Polak A, Pedzinska-Betiuk A, Weresa J, Malinowska B, Lewandowska A, et al. Fatty acid amide hydrolase inhibitor (URB597) as a regulator of myocardial lipid metabolism in spontaneously hypertensive rats. Chem Phys Lipids. 2019;218:141–8. https://doi.org/10.1016/j.chemphyslip.2018.12.007.
Schloss MJ, Horckmans M, Guillamat-Prats R, Hering D, Lauer E, Lenglet S, et al. 2-Arachidonoylglycerol mobilizes myeloid cells and worsens heart function after acute myocardial infarction. Cardiovasc Res. 2019;115(3):602–13. https://doi.org/10.1093/cvr/cvy242.
Rezkalla S, Kloner RA. Cardiovascular effects of marijuana. Trends Cardiovasc Med. 2019;29(7):403–7. https://doi.org/10.1016/j.tcm.2018.11.004.
Singh A, Saluja S, Kumar A, Agrawal S, Thind M, Nanda S, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7(1):45–59. https://doi.org/10.1007/s40119-017-0102-x.
Page RL, 2nd, Allen LA, Kloner RA, Carriker CR, Martel C, Morris AA et al. Medical marijuana, recreational cannabis, and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2020:CIR0000000000000883. https://doi.org/10.1161/CIR.0000000000000883.
Richards JR, Blohm E, Toles KA, Jarman AF, Ely DF, Elder JW. The association of cannabis use and cardiac dysrhythmias: a systematic review. Clin Toxicol (Phila). 2020:1–9. https://doi.org/10.1080/15563650.2020.1743847.
Pei SJ, Zhu HY, Guo JH, Zhang X, Deng ZJ. Knockout of CNR1 prevents metabolic stress-induced cardiac injury through improving insulin resistance (IR) injury and endoplasmic reticulum (ER) stress by promoting AMPK-alpha activation. Biochem Biophys Res Commun. 2018;503(2):744–51. https://doi.org/10.1016/j.bbrc.2018.06.070.
Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61(3):716–27. https://doi.org/10.2337/db11-0477.
Valenta I, Varga ZV, Valentine H, Cinar R, Horti A, Mathews WB, et al. Feasibility evaluation of myocardial cannabinoid type 1 receptor imaging in obesity: a translational approach. JACC Cardiovasc Imaging. 2018;11(2 Pt 2):320–32. https://doi.org/10.1016/j.jcmg.2017.11.019.
Martin Gimenez VM, Noriega SE, Kassuha DE, Fuentes LB, Manucha W. Anandamide and endocannabinoid system: an attractive therapeutic approach for cardiovascular disease. Ther Adv Cardiovasc Dis. 2018;12(7):177–90. https://doi.org/10.1177/1753944718773690.
Piscitelli F, Silvestri C. Role of the endocannabinoidome in human and mouse atherosclerosis. Curr Pharm Des. 2019;25(29):3147–64. https://doi.org/10.2174/1381612825666190826162735.
Rajesh M, Mukhopadhyay P, Hasko G, Pacher P. Cannabinoid CB1 receptor inhibition decreases vascular smooth muscle migration and proliferation. Biochem Biophys Res Commun. 2008;377(4):1248–52. https://doi.org/10.1016/j.bbrc.2008.10.159.
Molica F, Burger F, Thomas A, Staub C, Tailleux A, Staels B, et al. Endogenous cannabinoid receptor CB1 activation promotes vascular smooth-muscle cell proliferation and neointima formation. J Lipid Res. 2013;54(5):1360–8. https://doi.org/10.1194/jlr.M035147.
Schwartz M, Bockmann S, Hinz B. Up-regulation of heme oxygenase-1 expression and inhibition of disease-associated features by cannabidiol in vascular smooth muscle cells. Oncotarget. 2018;9(77):34595–616. https://doi.org/10.18632/oncotarget.26191.
Remiszewski P, Jarocka-Karpowicz I, Biernacki M, Jastrzab A, Schlicker E, Toczek M et al. Chronic cannabidiol administration fails to diminish blood pressure in rats with primary and secondary hypertension despite its effects on cardiac and plasma endocannabinoid system, oxidative stress and lipid metabolism. Int J Mol Sci. 2020;21(4). https://doi.org/10.3390/ijms21041295.
Duerr GD, Heinemann JC, Kley J, Eichhorn L, Frede S, Weisheit C, et al. Myocardial maladaptation to pressure overload in CB2 receptor-deficient mice. J Mol Cell Cardiol. 2019;133:86–98. https://doi.org/10.1016/j.yjmcc.2019.06.003.
Duerr GD, Feisst A, Halbach K, Verfuerth L, Gestrich C, Wenzel D, et al. CB2-deficiency is associated with a stronger hypertrophy and remodeling of the right ventricle in a murine model of left pulmonary artery occlusion. Life Sci. 2018;215:96–105. https://doi.org/10.1016/j.lfs.2018.11.003.
Miranda K, Mehrpouya-Bahrami P, Nagarkatti PS, Nagarkatti M. Cannabinoid receptor 1 blockade attenuates obesity and adipose tissue type 1 inflammation through miR-30e-5p regulation of delta-like-4 in macrophages and consequently downregulation of Th1 cells. Front Immunol. 2019;10:1049. https://doi.org/10.3389/fimmu.2019.01049.
Lahesmaa M, Eriksson O, Gnad T, Oikonen V, Bucci M, Hirvonen J, et al. Cannabinoid type 1 receptors are upregulated during acute activation of Brown adipose tissue. Diabetes. 2018;67(7):1226–36. https://doi.org/10.2337/db17-1366.
Eid BG. Cannabinoids for treating cardiovascular disorders: putting together a complex puzzle. J Microsc Ultrastruct. 2018;6(4):171–6. https://doi.org/10.4103/JMAU.JMAU_42_18.
Fulmer ML, Thewke DP. The Endocannabinoid system and heart disease: the role of cannabinoid receptor type 2. Cardiovasc Hematol Disord Drug Targets. 2018;18(1):34–51. https://doi.org/10.2174/1871529X18666180206161457.
Palomares B, Ruiz-Pino F, Garrido-Rodriguez M, Eugenia Prados M, Sanchez-Garrido MA, Velasco I, et al. Tetrahydrocannabinolic acid A (THCA-A) reduces adiposity and prevents metabolic disease caused by diet-induced obesity. Biochem Pharmacol. 2020;171:113693. https://doi.org/10.1016/j.bcp.2019.113693.
Carmona-Hidalgo B G-MI, García-Martín A, Ruiz-Pino F, Appendino G, Tena-Sempere M, Muñoz E. Δ9-Tetrahydrocannabinolic acid markedly alleviates liver fibrosis and inflammation in murine models of chemically- and obesity-induced liver injury. bioRxi 2020. https://doi.org/10.1101/2020.05.11.088070.
Morales P, Reggio PH, Jagerovic N. An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front Pharmacol. 2017;8:422. https://doi.org/10.3389/fphar.2017.00422.
Russo EB, Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. Adv Pharmacol. 2017;80:67–134. https://doi.org/10.1016/bs.apha.2017.03.004.
Youssef DA, El-Fayoumi HM, Mahmoud MF. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: involvement of CB2 and PPAR-gamma receptors. Chem Biol Interact. 2019;297:16–24. https://doi.org/10.1016/j.cbi.2018.10.010.
Nikolic D, Castellino G, Banach M, Toth PP, Ivanova E, Orekhov AN, et al. PPAR agonists, atherogenic dyslipidemia and cardiovascular risk. Curr Pharm Des. 2017;23(6):894–902. https://doi.org/10.2174/1381612822666161006151134.
Palomares B, Ruiz-Pino F, Navarrete C, Velasco I, Sanchez-Garrido MA, Jimenez-Jimenez C, et al. VCE-004.8, A multitarget cannabinoquinone, attenuates adipogenesis and prevents diet-induced obesity. Sci Rep. 2018;8(1):16092. https://doi.org/10.1038/s41598-018-34259-0.
Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225(3):631–7. https://doi.org/10.1002/jcp.22322.
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118(6):1021–40. https://doi.org/10.1161/CIRCRESAHA.115.306565.
Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103(20):7895–900. https://doi.org/10.1073/pnas.0511232103.
Xu Z, Mueller RA, Park SS, Boysen PG, Cohen MV, Downey JM. Cardioprotection with adenosine A2 receptor activation at reperfusion. J Cardiovasc Pharmacol. 2005;46(6):794–802. https://doi.org/10.1097/01.fjc.0000188161.57018.29.
Kitsis RN, Jialal I. Limiting myocardial damage during acute myocardial infarction by inhibiting C-reactive protein. N Engl J Med. 2006;355(5):513–5. https://doi.org/10.1056/NEJMcibr063197.
Lee WS, Erdelyi K, Matyas C, Mukhopadhyay P, Varga ZV, Liaudet L, et al. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: implications to autoimmune disorders and organ transplantation. Mol Med. 2016;22:136–46. https://doi.org/10.2119/molmed.2016.00007.
Walsh SK, Hepburn CY, Kane KA, Wainwright CL. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br J Pharmacol. 2010;160(5):1234–42. https://doi.org/10.1111/j.1476-5381.2010.00755.x.
Logue SE, Gustafsson AB, Samali A, Gottlieb RA. Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol. 2005;38(1):21–33. https://doi.org/10.1016/j.yjmcc.2004.11.009.
Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56(25):2115–25. https://doi.org/10.1016/j.jacc.2010.07.033.
Casares L, Garcia V, Garrido-Rodriguez M, Millan E, Collado JA, Garcia-Martin A, et al. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020;28:101321. https://doi.org/10.1016/j.redox.2019.101321.
Yano Y, Ozono R, Oishi Y, Kambe M, Yoshizumi M, Ishida T, et al. Genetic ablation of the transcription repressor Bach1 leads to myocardial protection against ischemia/reperfusion in mice. Genes Cells. 2006;11(7):791–803. https://doi.org/10.1111/j.1365-2443.2006.00979.x.
Omura S, Suzuki H, Toyofuku M, Ozono R, Kohno N, Igarashi K. Effects of genetic ablation of bach1 upon smooth muscle cell proliferation and atherosclerosis after cuff injury. Genes Cells. 2005;10(3):277–85. https://doi.org/10.1111/j.1365-2443.2005.00832.x.
Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry LJ, Kundu A, et al. The general anesthetic propofol increases brain N-arachidonylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase. Br J Pharmacol. 2003;139(5):1005–13. https://doi.org/10.1038/sj.bjp.0705334.
Sun HJ, Lu Y, Wang HW, Zhang H, Wang SR, Xu WY, et al. Activation of endocannabinoid receptor 2 as a mechanism of propofol pretreatment-induced cardioprotection against ischemia-reperfusion injury in rats. Oxidative Med Cell Longev. 2017;2017:2186383–18. https://doi.org/10.1155/2017/2186383.
Matyas C, Erdelyi K, Trojnar E, Zhao S, Varga ZV, Paloczi J, et al. Interplay of liver-heart inflammatory axis and cannabinoid 2 receptor signaling in an experimental model of hepatic cardiomyopathy. Hepatology. 2020;71(4):1391–407. https://doi.org/10.1002/hep.30916.
Garcia-Martin A, Garrido-Rodriguez M, Navarrete C, Caprioglio D, Palomares B, DeMesa J, et al. Cannabinoid derivatives acting as dual PPARgamma/CB2 agonists as therapeutic agents for systemic sclerosis. Biochem Pharmacol. 2019;163:321–34. https://doi.org/10.1016/j.bcp.2019.02.029.
Garcia-Martin A, Garrido-Rodriguez M, Navarrete C, Del Rio C, Bellido ML, Appendino G, et al. EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis. Biochem Pharmacol. 2018;157:304–13. https://doi.org/10.1016/j.bcp.2018.07.047.
Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350(2–3):240–4. https://doi.org/10.1016/0014-5793(94)00773-x.
Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. 2009;119(1):2836–6. https://doi.org/10.1161/CIRCULATIONAHA.108.811992.
Jones D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov. 2008;7(12):961–2. https://doi.org/10.1038/nrd2775.
Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205(1):171–4. https://doi.org/10.1007/s00213-009-1506-7.
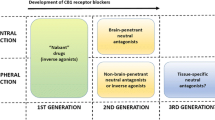
Cinar R, Iyer MR, Kunos G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol Ther. 2020;208:107477. https://doi.org/10.1016/j.pharmthera.2020.107477.
Takano A, Gulyas B, Varnas K, Little PB, Noerregaard PK, Jensen NO, et al. Low brain CB1 receptor occupancy by a second generation CB1 receptor antagonist TM38837 in comparison with rimonabant in nonhuman primates: a PET study. Synapse. 2014;68(3):89–97. https://doi.org/10.1002/syn.21721.
Klumpers LE, Fridberg M, de Kam ML, Little PB, Jensen NO, Kleinloog HD, et al. Peripheral selectivity of the novel cannabinoid receptor antagonist TM38837 in healthy subjects. Br J Clin Pharmacol. 2013;76(6):846–57. https://doi.org/10.1111/bcp.12141.
Micale V, Drago F, Noerregaard PK, Elling CE, Wotjak CT. The cannabinoid CB1 antagonist TM38837 with limited penetrance to the brain shows reduced fear- promoting effects, in mice. Front Pharmacol. 2019;10:207. https://doi.org/10.3389/fphar.2019.00207.
Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–79. https://doi.org/10.1016/j.cmet.2012.07.002.
Liu J, Godlewski G, Jourdan T, Liu Z, Cinar R, Xiong K, et al. Cannabinoid-1 receptor antagonism improves glycemic control and increases energy expenditure through sirtuin-1/mechanistic target of rapamycin complex 2 and 5′adenosine monophosphate-activated protein kinase signaling. Hepatology. 2019;69(4):1535–48. https://doi.org/10.1002/hep.30364.
Stasiulewicz A, Znajdek K, Grudzien M, Pawinski T, Sulkowska AJI. A guide to targeting the endocannabinoid system in drug design. Int J Mol Sci. 2020;21(8). https://doi.org/10.3390/ijms21082778.
Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153(9):183746–1846. https://doi.org/10.1016/j.pain.2012.04.020.
Postnov A, Schmidt ME, Pemberton DJ, de Hoon J, van Hecken A, van den Boer M, et al. Fatty acid amide hydrolase inhibition by JNJ-42165279: a multiple-ascending dose and a positron emission tomography study in healthy volunteers. Clin Transl Sci. 2018;11(4):397–404. https://doi.org/10.1111/cts.12548.
Kerbrat A, Ferre JC, Fillatre P, Ronziere T, Vannier S, Carsin-Nicol B, et al. Acute neurologic disorder from an inhibitor of fatty acid amide hydrolase. N Engl J Med. 2016;375(18):1717–25. https://doi.org/10.1056/NEJMoa1604221.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and Metabolic Syndrome
Rights and permissions
About this article
Cite this article
Navarrete, C., Garcia-Martin, A., DeMesa, J. et al. Cannabinoids in Metabolic Syndrome and Cardiac Fibrosis. Curr Hypertens Rep 22, 98 (2020). https://doi.org/10.1007/s11906-020-01112-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11906-020-01112-7