Abstract
Purpose of Review
Previous trials definitively established that lowering systolic blood pressure (BP) to 140 mmHg prevented heart failure (HF) exacerbations, but the potential benefits and risks of further BP reduction remain unclear due to a paucity of trial-based data.
Recent Findings
A recent secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) found that in older, high-risk, non-diabetic participants with systolic hypertension, a BP treatment target < 120 mmHg resulted in a 36% lower rate of acute decompensated HF as compared with a BP target < 140 mmHg. Those participants with incident HF had a 26-fold increased risk of subsequent cardiovascular events and death. Based in part on the SPRINT results, the 2017 American Heart Association/American College of Cardiology/HF Society Guideline for the Management of HF acknowledged that targeting a significant reduction in BP in those at increased risk for cardiovascular disease is a novel risk-based strategy to prevent HF.
Summary
SPRINT redefines systolic BP target goals in older, high-risk patients and provides a key opportunity for preventing HF in this patient group.
Similar content being viewed by others
Introduction
Hypertension (HTN) remains a major public health problem associated with considerable morbidity and mortality. HTN continues to be the most prevalent risk factor for heart failure (HF) and precedes the diagnosis of HF in 75–85% of persons who develop HF [1, 2]. Higher systolic blood pressure (SBP) increases the risk of developing HF, and BP reduction prevents incident HF, but the optimal BP target for prevention of HF remains uncertain [3]. Further, in the elderly, aggressive BP-lowering strategies may potentially lead to complications, such as mechanical falls with injury and renal failure, as well as adverse effects associated with polypharmacy. This article aims to review current BP targets to prevent HF among older patients with HTN.
Case Histories
Patient 1: an 84-year-old African American man (body mass index (BMI) of 34) who is followed routinely at his cardiologist’s clinic subsequent to a coronary revascularization performed 5 years ago. He remains asymptomatic without diabetes mellitus (DM), but continues to smoke half-a-pack of cigarettes per day. His last echocardiogram showed a normal left ventricular ejection fraction (LVEF) 1 year ago. His routine laboratory tests showed an estimated creatinine clearance of 35 ml/min. His BP was 140/90 mmHg after 5 min in a seated position.
Patient 2: an 84-year-old Caucasian woman (BMI, 24) was examined at a routine annual visit with her primary care physician. She was asymptomatic. Her BP was 140/90 mmHg after 5 min in a seated position. She did not have DM or history of cardiovascular disease (CVD). Her routine laboratory tests were unremarkable, including normal renal function.
To reduce the risk of HF, should both patients be treated to a BP reduction target of < 120/80 mmHg?
Hypertension and HF Risk—Pathophysiology
The progression from HTN to structural cardiac changes and eventually systolic and diastolic left ventricular (LV) dysfunction is demonstrated in Fig. 1. Although LV hypertrophy (LVH) can precede the development of HTN, the progression from HTN to concentric LVH is an important step in the pathway toward HF. Along with mechanical stress resulting from pressure overload, neurohormonal abnormalities also play an important role in LVH. Neurohormones can directly promote myocyte hypertrophy and matrix deposition independently of their effects on BP [4]. There is a considerable inter-individual variability in how the LV hypertrophies in response to HTN. For example, compared to Caucasians, African Americans have higher LV mass, are more likely to develop concentric hypertrophy, and experience more severe diastolic dysfunction [5,6,7]. Similarly, those with higher SBP develop concentric hypertrophy much more frequently than eccentric hypertrophy [8]. Women with isolated systolic HTN also develop concentric LVH [9]. Increasing age has also been associated with a concentric as opposed to an eccentric hypertrophic response [1]. Along with afterload excess and LVH with its associated cardiac fibrosis and increased arterial stiffness, HTN also induces inflammation, oxidative stress, and endothelial dysfunction—all predispositions to HF [10]. Further, HTN may progress directly to HF in the absence of LVH or myocardial ischemia or infarction. However, contrary to conventional belief, BP may account for only 25% of the variability of LV mass in a population [11]. Indeed, the majority of patients with HF with preserved EF did not have significant LVH at baseline [12]. A recent report by Soliman et al. showed that changes in electrocardiographic LVH explained only 1% of the reduction in CVD events in the Systolic Blood Pressure Intervention Trial (SPRINT) [13]. Thus, there is uncertainty regarding the relationships between BP lowering, LV mass reduction, and improved CVD outcomes in hypertensive patients, particularly at the lower ranges of target BP.
Systolic Blood Pressure Target and HF Risk
Risk for HF rises continuously with increasing BP [3]. The lifetime risk for HF doubles in those with BP > 160/100 versus < 140/90 mmHg [14]. Several prior trials in older patients with systolic HTN showed large reductions in new HF events resulting from SBP reductions to 140–145 mmHg [15,16,17,18] (Table 1). The particularly large reduction in HF events in the Hypertension in the Very Elderly Trial (HYVET) likely reflects the older age of the participants compared to the other three trials [15]. Similarly, a larger benefit was also observed in participants aged > 80 years in the Systolic Hypertension in the Elderly Program (SHEP) trial [16]. Although the benefit of lowering SBP to 140 mmHg for preventing HF events was well established by previous trials [15,16,17,18], there has been a paucity of information regarding the potential benefit and risk of lowering BP further. To address this uncertainty, a propensity score analysis of 7785 patients with mild to moderate HF with reduced or preserved EF followed for 5 years was carried out. The study found that a baseline SBP ≤ 120 mmHg was associated with increased CV and HF mortality and all-cause, CV, and HF hospitalizations, independently of other baseline characteristics [24]. Similarly, BP-lowering therapy among intermediate-risk adults showed a trend for harm among those with baseline SBP levels < 130 mmHg in the Heart Outcomes Prevention Evaluation (HOPE-3) trial [25]. Achieving intensive SBP reductions will inevitably also lower diastolic BP (DBP). Since myocardial perfusion requirements are increased in HTN, and myocardial perfusion pressure depends on adequate DBP, a drop in DBP could result in myocardial ischemia and increase LV dilation with subsequent HF with reduced LVEF [26]. A recent study demonstrated that among adults with a systolic BP ≥ 120 mmHg, a low DBP, particularly < 60 mmHg, was associated with subclinical myocardial damage and coronary artery disease events [27].
Intensive Systolic Blood Pressure Target (< 130 mmHg) and HF
Based on data to this point, the outcomes from large clinical trials have not successfully addressed the question of whether lowering SBP < 130 mmHg is an effective strategy to prevent HF. The Studio Italiano Sugli Effetti CARDIOvascolari del Controllo della Pressione Arteriosa SIStolica (Cardio-Sis) trial showed that lowering systolic BP to < 130 mmHg in non-diabetic patients decreased composite CV outcomes compared with a SBP < 140 mmHg [20]. However, HF event reduction was not significantly different between treatment arms (hazard ratio (HR), 0.42; 95% confidence interval (CI), 0.11–1.63) (Table 1). The results of the Cardio-Sis trial have to be interpreted within the context of its potential limitations. First of all, they powered their study on LVH as the primary outcome. Few clinical events, short clinical follow-up time with a fairly small sample size might have affected the power to examine HF outcomes. Cardio-Sis excluded people with DM and chronic kidney disease (CKD). In addition, the study included only Caucasian patients, so extrapolation to other racial/ethnic groups might not be justified. The study was not double-blind; thus, awareness of the randomization code could have affected the clinical decisions related to admission for HF events [20].
In ACCORD (Action to Control Cardiovascular Risk in Diabetes), a large randomized trial that specifically addressed the potential benefit of lowering SBP to < 130 mmHg (the target was 120 mmHg) in patients with DM, the HF event reduction was smaller and not statistically significant (HR, 0.94; 95% CI, 0.70–1.26) [19] (Table 1). This lower event rate in ACCORD was likely because of several factors. ACCORD recruited patients with DM and excluded people with CKD and those aged > 79 years. In addition, inclusion criteria directed participants with dyslipidemia into the ACCORD lipid trial, leaving participants who were at lower risk for CV events to be enrolled into the BP trial. ACCORD also used a factorial design that included comparisons of standard and intensive glycemic and lipid treatment targets in the same trial. Furthermore, the event rate in the standard therapy group in ACCORD was almost 50% lower than expected; thus, the trial may not have been adequately powered to examine HF events. In a recent meta-analysis, every 10-mmHg reduction in SBP reduced the risk of HF by an average of 28% (HR, 0.72; 95% CI, 0.67–0.78; p < 0.001). The proportional reductions per 10-mmHg decrease in SBP were greater for stroke and HF than for coronary heart disease, and there was a trend toward decreased HF events even with baseline SBP < 130 mmHg [28]. Similarly, meta-analysis of 35 HTN treatment trials with HF events showed a strong, significant correlation between the extent of SBP and DBP reduction and the reduction in HF events [29].
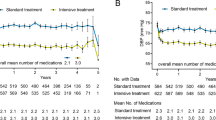
A secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT), a large multicenter (102 sites), racially diverse randomized open-label trial, showed that treatment that targets a SBP of < 120 mmHg, compared with < 140 mmHg, resulted in a 36% lower rate of acute decompensated HF events [22•] (Table 1). Persons with DM, those with a history of stroke, and institutionalized people were excluded from the study. Symptomatic HF within the past 6 months, a LVEF of less than 35%, and an estimated glomerular filtration rate less than 20 ml/min/1.73 were also exclusions [21•]. All HF events were new (incident) events and were adjudicated based on a manual of operations that had been validated in the Atherosclerosis Risk in Communities (ARIC) study [30]. The beneficial effect of the intervention on the HF event rate became apparent early, at 6-month follow-up, and increased with duration of follow-up [22•]. The beneficial effect was consistent across all the key pre-specified subgroups, including age > 75 years or < 75 years, with or without prior CVD, with or without CKD, women or men, black race or non-black race, and the tertiles of baseline SBP [22•]. Participants who had an initial HF event had markedly increased risk of subsequent events, including recurrent HF (Fig. 2) [22•]. Similar results were also seen in the SPRINT SENIORS cohort (participants’ age ≥ 75 years) [23•].
Clinical Implications of Lowering SBP to < 130 mmHg: Feasibility, Safety, and Patient Burden
While the efficacy of the SPRINT strategy is clear, given that the trial was stopped early due to benefit, some have questioned the feasibility, safety, and patient burden of lowering SBP to < 130 mmHg, particularly in older, frail patients. However, in both the main SPRINT and in the SPRINT SENIORS cohort, HF events were lower in the intensive arm compared with those of the standard arm, despite significantly lower DBPs (SPRINT, 69 versus 76 mmHg; SPRINT SENIORS, 62 versus 67 mmHg) [21•, 23•]. The benefit of intensive BP control was consistent among elderly persons (≥ 75 years) who were frail or had reduced gait speed [23•]. An analysis of the HYVET population showed similar treatment benefits, even in the frailest participants [31]. Furthermore, the overall serious adverse event rate was comparable in both treatment groups, including among the frailest participants in the SPRINT SENIORS cohort [23•]. There were no differences between treatment groups in injurious falls or orthostatic hypotension [23•]. Similarly, the ACCORD trial showed that intensive treatment (mean SBP < 120 mmHg) was not associated with an increased risk of falls or non-spine fractures in patients with type II DM [32]. Further, the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston (MOBILIZE Boston Study) showed that improved BP control (< 140/90 mmHg) reduces risk for orthostatic hypotension in older community-dwelling adults (mean age of 78 years; female, 65%) and has no effect on risk for injurious falls [33]. A recent meta-analysis of existing randomized trials suggested that in patients with HTN, an on-treatment SBP target of < 130 mmHg achieved optimal balance between efficacy and safety [34]. Although there is no evidence of permanent kidney injury associated with the lower BP goal in SPRINT SENIORS, mild acute kidney injury occurred more frequently in the intensive treatment group [23•]. Similarly, hypotension, electrolyte abnormalities, and syncope were more frequent in the intensive group, though infrequent in the study overall [21•]. In the SPRINT intensive treatment group, an average of 2.8 antihypertensive drugs was required to reach SBP goal. Some healthcare providers have expressed reluctance to prescribe more than two antihypertensive drugs to a given patient, and adherence is generally lower with increasing complexity of clinical regimens. However, these disadvantages must be balanced with the clear benefit of substantially reduced mortality and CVD events from adopting the SPRINT intensive BP treatment strategy.
What Does the SPRINT Add?
The SPRINT results have substantial implications for the future of intensive BP therapy in older adults because of this condition’s high prevalence, the high absolute risk for CVD complications from elevated BP, and the devastating consequences of such events on the independent function of older people. However, the public health implications are dependent on the generalizability of the SPRINT outcomes to the US population, especially populations excluded from the trial, e.g., younger and lower-risk persons; those with DM, severe kidney disease, prior HF, and stroke; and subgroups of elderly adults (nursing home residents, extremely frail or demented individuals). Using data from the National Health and Nutrition Examination Survey (NHANES), Bress et al. found that 8.2 million adults with treated HTN (17% of the hypertensive population) meet the SPRINT eligibility criteria and thus may benefit from intensive BP treatment [35]. They also predicted that in patients who fit SPRINT eligibility criteria, intensive BP treatment would prevent approximately 46,100 cases of incident HF per year but would cause 56,100 episodes of hypotension, 88,700 cases of AKI, 34,400 episodes of syncope, and 43,400 cases of electrolyte disorders (hyponatremia and hypokalemia) compared to standard care [36].
Blood Pressure Measurement in SPRINT
Knowing how BP is measured is important for guiding clinicians in appropriate management of HTN [37]. Although numerous HTN experts have argued that the BP measurement technique in SPRINT makes it an outlier, SPRINT BP measurements were conducted using methods that were commonly recommended by professional societies and BP guideline committees [38•, 39]. The SPRINT used programmable automated oscillometric devices (Omron Digital BP Monitor) to measure BP [40]. This device could be programed to incorporate the 5-min rest and then initiate the three BP measurements automatically after the 5 min had elapsed. Coordinators were instructed how to program the Omron device during training [40]. The coordinators could have been in or out of the room during the 5-min rest period and/or during the time the Omron was automatically taking the BP measurements. Recent publications have stated that the BP measurement technique used in SPRINT was unattended, and was not comparable with BP readings in other trials where the measurement was attended and that the intensive treatment goal of < 120 mmHg in SPRINT would actually correspond to higher SBP values in other trials [41]. Notably, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines do not comment on the presence or absence of an observer during BP measurement. The recent post hoc SPRINT analysis suggested that there was no compelling evidence in SPRINT that unattended BP measurements led to lower SBP at baseline or during follow-up compared to the attended BP measurements [40]. Importantly, similar BP levels and CVD risk reduction were observed in the intensive treatment group of SPRINT participants whether the measurement technique used was primarily attended or unattended [40]. Similarly, data from the SPRINT Ambulatory BP Ancillary Study also showed that the BP values obtained at the SPRINT study clinic visit, whether attended or unattended, are similar to values obtained during 24-h ambulatory BP monitoring [42].
Impact of SPRINT on Guidelines
In 2017, the ACC/AHA HTN guideline changed the definition of HTN to incorporate the former “pre-hypertension” as stage 1 hypertension. Thus, normal BP is considered < 120/80 mmHg, elevated BP is 120–129/80 mmHg, and hypertension is > 130/80 mmHg [38•]. Similarly, they recommended that in adults with HTN and increased risk of HF, the optimal BP should be < 130/80 mmHg [38•]. The guideline committee concluded that the available randomized controlled trials that provided evidence for their recommendation were efficacy studies in which BP measurements were more consistent with guideline recommendations than is common in clinical practice, resulting in lower absolute values for SBP. However, the Eighth Joint National Committee determined that SBP targets should be below 140 mmHg or below 150 mmHg in those 60 years of age or older [39]. The 2016 Canadian HTN Education Program Guidelines recommend intensive BP treatment with target SBP ≤ 120 mmHg (grade B) for high-risk patients based on automated office BP measurements (grade D) [43•]. Importantly, the 2016 Canadian HTN Education Program Guidelines recommend that BP be measured as in the SPRINT. The 2016 Australian guidelines recommend a SBP target < 120 mmHg (strong recommendation, class II) for high-CV-risk patients without DM, including CKD patients and those aging > 75 years [44]. Finally, the 2017 ACC/AHA HF guideline is one of the first to recommend the lower SBP target of 130 mmHg to prevent HF, based in part on the results of the SPRINT [21•, 22•, 23•, 45•].
Case Resolution
Based on this, considering that the risk for future development of HF differs considerably among these individuals, should their therapeutic targets be different? Would a lower SBP target for patient 1 than current recommendation further decrease the risk of HF? It is self-evident that the patient described in case 1 is at significantly higher risk of development of HF compared with patient 2. The former patient has a history of CVD and renal dysfunction. Patient 1 definitely needs a SBP target of 130 mmHg. If we implement the guideline-recommended BP measurement technique as in SPRINT, patient 1 needs a SBP target of 120 mmHg. However, for patient 2, the SBP target to reduce HF risk remains uncertain. On the basis of the available data, we recommend the SBP target of 2 to 130 mmHg for patient 2 to reduce the risk of HF.
In summary, using the SPRINT intensive treatment algorithm and a SBP goal of < 120 mmHg, along with the BP measurement techniques recommended by HTN guideline committees (staff training to allow for a quiet rest period, proper positioning of the arm and body, use of proper cuff size, and multiple measurements using a validated automated BP device), will reduce the risks of HF in non-diabetic patients at medium–high CVD risk.
Conclusion
Uncontrolled SBP continues to be a highly prevalent and highly modifiable HF risk factor. Targeting only those at the highest end of the BP spectrum does not address most individuals at risk for developing HF. Therefore, treatment decisions should be based on a person’s absolute risk. The results of SPRINT are likely to have a major impact on the treatment of HTN. SPRINT results are reflected in changes in recent HTN guidelines regarding treatment goals and BP measurement techniques. SPRINT revisits BP target goals and challenges us to improve BP measurement and management to prevent HF events. In addition, these results suggest that translation of the SPRINT results will require measurement of BP as performed in that trial. After all, BP is a vital sign and should be measured as in the clinical trials so that we can provide evidence-based care to our patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9.
Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37.
Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62.
Johnson DB, Dell’Italia LJ. Cardiac hypertrophy and failure in hypertension. Curr Opin Nephrol Hypertens. 1996;5:186–91.
Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network Study. Hypertension. 2004;43:1182–8.
Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–9.
Sharp A, Tapp R, Francis DP, McG Thom SA, Hughes AD, Stanton AV, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015–21.
Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–8.
Krumholz HM, Larson MG, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–84.
Cohen RA, Tong X. Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease. J Cardiovasc Pharmacol. 2010;55:308–16.
de Simone G, Devereux RB, Izzo R, Girfoglio D, Lee ET, Howard BV, et al. Lack of reduction of left ventricular mass in treated hypertension: the strong heart study. J Am Heart Assoc. 2013;2:e000144.
Solomon S, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–95.
Soliman EZ, Ambrosius WT, Cushman WC, Zhang ZM, Bates JT, Neyra JA, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation. 2017;136:440–50.
Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72.
Beckett S, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98.
Kostis J, Davis BR, Cutler JA, Grimm RH Jr, Berge KG, Cohen JD, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1997;278:212–6.
Stassen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–64.
The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–97.
ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;363:1563–74.
Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–33.
• Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16 This multicenter randomized trial of intensive (target < 120/80 mmHg) compared to standard (target < 140/90 mmHg) hypertension treatment in high-risk, non-diabetic patients, showed significant reduction in the primary composite endpoint and in all-cause mortality with intensive treatment.
• Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, et al. Effect of intensive blood pressure treatment on heart failure events in the systolic blood pressure reduction intervention trial. Circ Heart Fail. 2017;10:e003613 A secondary analysis from SPRINT focused on incident heart failure in 6 pre-specified subgrops, and showed significant reduction in risk of incident heart failure with intensive treatment.
• Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA. 2016;315:2673–82 A secondary analysis from SPRINT, focusing in participants over 75 years of age at enrollment, showed consistent benefit of intensive treatment for the primary composite outcome and for indicent heart failure. In addition, exploratory analysis suggested that the benefit of intensive BP control was consistent among elderly persons (≥ 75 years) who were frail or had reduced gait speed.
Banach M, Bhatia V, Feller MA, Mujib M, Desai RV, Ahmed MI, et al. Relation of baseline systolic blood pressure and long-term outcomes in ambulatory patients with chronic mild to moderate heart failure. Am J Cardiol. 2011;107:1208–14.
Lonn EM, Bosch J, Lopez-Jaramillo P, Zhu J, Liu L, Pais P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009–20.
Messerli FH, Panjrath GS. The J-curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54:1827–34.
McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;68:1713–22.
Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure - meta-analyses of randomized trials. J Hypertens. 2016;34:373–84.
Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9.
Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78.
Margolis KL, Palermo L, Vittinghoff E, Evans GW, Atkinson HH, Hamilton BP, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med. 2014;29:1599–606.
Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–9.
Bangalore S, Toklu B, Gianos E, Schwartzbard A, Weintraub H, Ogedegbe G, et al. Optimal systolic blood pressure target after SPRINT: insights from a network meta-analysis of randomized trials. Am J Med. 2017;130:707–19.
Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of SPRINT results to the U.S. adult population. J Am Coll Cardiol. 2016;67:463–72.
Bress AP, Kramer H, Khatib R, Beddhu S, Cheung AK, Hess R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: projections from NHANES (National Health and Nutrition Examination Survey). Circulation. 2017;135:1617–28.
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61.
• Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–324 Guideline document from the American College of Cardiology and the American Heart Association with proposed diagnostic and therapeutic strategies for hypertension.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20.
Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, et al. Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71:848–57.
Kjeldsen SE, Lund-Johansen P, Nilsson PM, Mancia G. Unattended blood pressure measurements in the systolic blood pressure intervention trial: implications for entry and achieved blood pressure values compared with other trials. Hypertension. 2016;67:808–12.
Drawz PE, Pajewski NM, Bates JT, Bello NA, Cushman WC, Dwyer JP, et al. Effect of intensive versus standard clinic-based hypertension management on ambulatory blood pressure: results from the SPRINT (Systolic Blood Pressure Intervention Trial) ambulatory blood pressure study. Hypertension. 2017;69:42–50.
Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569–88.
National Heart Foundation of Australia. Guideline for the diagnosis and management of hypertension in adults - 2016. Melbourne: National Heart Foundation of Australia, 2016; 2016. Ref Type: Generic
• Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the management of heart failure. J Am Coll Cardiol. 2017;70:776–803 Acknowledged that targeting a significant reduction in BP in those at increased risk for CVD is a novel risk-based strategy to prevent HF. The guideline states that in hypertensive patients at increased risk (stage A HF), optimal BP is < 130/80 mmHg. This recommendation reflects the assumption that BP measurements taken in the office setting are typically 5–10 mmHg higher than research-based measurements, as in SPRINT, so the < 130/80 mmHg goal is an approximation of the SPRINT intensive target BP adapted for conventional practice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Kitzman declares the following relationships: consultant for Abbvie, Bayer, Merck, Medtronic, GSK, Relypsa, Regeneron, Merck, Corvia Medical, DCRI, and Actavis, research grant funding from Novartis, St. Luke’s Medical Center, and stock ownership in Gilead Sciences. Dr. Upadhya has received research funding from Novartis and Corvia.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and the Heart
Rights and permissions
About this article
Cite this article
Upadhya, B., Stacey, R.B. & Kitzman, D.W. Preventing Heart Failure by Treating Systolic Hypertension: What Does the SPRINT Add?. Curr Hypertens Rep 21, 9 (2019). https://doi.org/10.1007/s11906-019-0913-3
Published:
DOI: https://doi.org/10.1007/s11906-019-0913-3