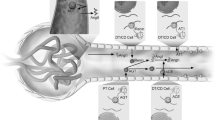
Abstract
Purpose of Review
The intrarenal renin-angiotensin-aldosterone system (RAS) is an independent paracrine hormonal system with an increasingly prominent role in hypertension and renal disease. Two enzyme components of this system are angiotensin-converting enzyme (ACE) and more recently discovered ACE2. The purpose of this review is to describe recent discoveries regarding the roles of intrarenal ACE and ACE2 and their interaction.
Recent Findings
Renal tubular ACE contributes to salt-sensitive hypertension. Additionally, the relative expression and activity of intrarenal ACE and ACE2 are central to promoting or inhibiting different renal pathologies including renovascular hypertension, diabetic nephropathy, and renal fibrosis.
Summary
Renal ACE and ACE2 represent two opposing axes within the intrarenal RAS system whose interaction determines the progression of several common disease processes. While this relationship remains complex and incompletely understood, further investigations hold the potential for creating novel approaches to treating hypertension and kidney disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–74.
• Yang T, Xu C. Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J Am Soc Nephrol. 2017;28:1040–9. https://doi.org/10.1681/ASN.2016070734. A recent review on the function and regulation of the intrarenal RAS in contrast with systemic RAS.
Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–9. https://doi.org/10.1681/ASN.2011121159.
Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Phys. 1997;272:F405–9.
Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002;13:2207–12.
• Carey RM. The intrarenal renin-angiotensin system in hypertension. Adv Chronic Kidney Dis. 2015;22:204–10. https://doi.org/10.1053/j.ackd.2014.11.004. A review of recent advances in understanding of the intrarenal RAS as well as the role of newer RAS pathways in hypertension.
Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–71. https://doi.org/10.1210/er.2003-0001.
Erdos EG, Skidgel RA. Structure and functions of human angiotensin I converting enzyme (kininase II). Biochem Soc Trans. 1985;13:42–4.
Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–23.
Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–90.
• Giani JF, Shah KH, Khan Z, Bernstein EA, Shen XZ, McDonough AA, et al. The intrarenal generation of angiotensin II is required for experimental hypertension. Curr Opin Pharmacol. 2015;21:73–81. https://doi.org/10.1016/j.coph.2015.01.002. This study shows that mice lacking renal ACE fail to generate intrarenal Ang II or develop hypertension when treated with Ang II or L-NAME.
Rahimi Z. The role of renin angiotensin aldosterone system genes in diabetic nephropathy. Can J Diabetes. 2016;40:178–83. https://doi.org/10.1016/j.jcjd.2015.08.016.
Bernstein KE, Giani JF, Shen XZ, Gonzalez-Villalobos RA. Renal angiotensin-converting enzyme and blood pressure control. Curr Opin Nephrol Hypertens. 2014;23:106–12. https://doi.org/10.1097/01.mnh.0000441047.13912.56.
Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, et al. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22:449–59. https://doi.org/10.1681/ASN.2010060624.
•• Giani JF, Eriguchi M, Bernstein EA, Katsumata M, Shen XZ, Li L, et al. Renal tubular angiotensin converting enzyme is responsible for nitro-L-arginine methyl ester (L-NAME)-induced salt sensitivity. Kidney Int. 2017;91:856–67. This study uses a mouse model with no renal tubular ACE and another with ACE only in the renal tubular epithelium to demonstrate that renal tubular ACE is critical for development of salt sensitivity in response to L-NAME.
Liebau MC, Lang D, Bohm J, Endlich N, Bek MJ, Witherden I, et al. Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol. 2006;290:F710–9.
Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, et al. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–7. https://doi.org/10.1152/ajprenal.00477.2009.
Vio CP, Jeanneret VA. Local induction of angiotensin-converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney Int Suppl. 2003;86:S57–63.
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9.
Padda RS, Shi Y, Lo CS, Zhang SL, Chan JS. Angiotensin-(1–7): a novel peptide to treat hypertension and nephropathy in diabetes? J Diabetes Metab. 2015;6. doi: https://doi.org/10.4172/2155-6156.1000615.
Shi Y, Lo CS, Padda R, Abdo S, Chenier I, Filep JG, et al. Angiotensin-(1-7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin-converting enzyme 2 and Mas receptor expression in diabetic mice. Clin Sci (Lond). 2015;128:649–63. https://doi.org/10.1042/CS20140329.
Xiao F, Burns KD. Measurement of angiotensin converting enzyme 2 activity in biological fluid (ACE2). Methods Mol Biol. 2017;1527:101–15. https://doi.org/10.1007/978-1-4939-6625-7_8.
Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204:587–93. https://doi.org/10.1002/path.1670.
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–9.
Osterreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, et al. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology. 2009;50:929–38. https://doi.org/10.1002/hep.23104.
Liu CX, Hu Q, Wang Y, Zhang W, Ma ZY, Feng JB, et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med. 2011;17:59–69. https://doi.org/10.2119/molmed.2010.00111.
• Callera GE, Antunes TT, Correa JW, Moorman D, Gutsol A, He Y, et al. Differential renal effects of candesartan at high and ultra-high doses in diabetic mice-potential role of the ACE2/AT2R/Mas axis. Biosci Rep. 2016;36:e00398. This study demonstrates that the renoprotective effects of the Candesartan occur with upregulation of the ACE2/AT2R/Mas axis.
• Huang YF, Zhang Y, Liu CX, Huang J, Ding GH. microRNA-125b contributes to high glucose-induced reactive oxygen species generation and apoptosis in HK-2 renal tubular epithelial cells by targeting angiotensin-converting enzyme 2. Eur Rev Med Pharmacol Sci. 2016;20:4055–62. This in vitro study shows that blocking microRNA-125b mediated downregulation of ACE2 in renal tubular epithelial cells and prevents high glucose-mediated ROS production and apoptosis.
• Berger RC, Vassallo PF, Crajoinas Rde O, Oliveira ML, Martins FL, Nogueira BV, et al. Renal effects and underlying molecular mechanisms of long-term salt content diets in spontaneously hypertensive rats. PLoS One. 2015;10:e0141288. https://doi.org/10.1371/journal.pone.0141288. This study describes the effects of high- and low-salt diets on ACE/ACE2 ratio and associated renal injury.
Chan J, Lo CS, Shi Y, Chenier I, Zhang SL. Os 25-03 overexpression of heterogeneous nuclear ribonucleoprotein F prevents systemic hypertension and kidney injury and normalizes renal renin-angiotensin system genes expression in type 2 diabetic Db/db transgenic mice. J Hypertens. 2016;34(Suppl 1 - ISH 2016 Abstract Book):e245. https://doi.org/10.1097/01.hjh.0000500552.89974.b8.
Bae EH, Konvalinka A, Fang F, Zhou X, Williams V, Maksimowski N, et al. Characterization of the intrarenal renin-angiotensin system in experimental Alport syndrome. Am J Pathol. 2015;185:1423–35. https://doi.org/10.1016/j.ajpath.2015.01.021.
Ross MJ, Nangaku M. ACE2 as therapy for glomerular disease: the devil is in the detail. Kidney Int. 2017;91:1269–71.
• Chen LJ, Xu YL, Song B, Yu HM, Oudit GY, Xu R, et al. Angiotensin-converting enzyme 2 ameliorates renal fibrosis by blocking the activation of mTOR/ERK signaling in apolipoprotein E-deficient mice. Peptides. 2016;79:49–57. https://doi.org/10.1016/j.peptides.2016.03.008. This study demonstrates that recombinant ACE2 prevents Ang II-mediated fibrosis in ApoE knockout mice via upregulation Ang(1–7) and inhibition of mTOR/ERK.
• Le Y, Zheng Z, Xue J, Cheng M, Guan M, Xue Y. Effects of exendin-4 on the intrarenal renin-angiotensin system and interstitial fibrosis in unilateral ureteral obstruction mice: exendin-4 and unilateral ureteral obstruction. J Renin-Angiotensin-Aldosterone Syst. 2016;17:1470320316677918. This study demonstrates that exendin-4 reduces renal fibrosis in the setting of ureteral obstruction while increasing ACE2 and decreasing ACE expressions.
Rodriguez-Iturbe B, Franco M, Johnson RJ. Impaired pressure natriuresis is associated with interstitial inflammation in salt-sensitive hypertension. Curr Opin Nephrol Hypertens. 2013;22:37–44. https://doi.org/10.1097/MNH.0b013e32835b3d54.
•• Giani JF, Bernstein KE, Janjulia T, Han J, Toblli JE, Shen XZ, et al. Salt sensitivity in response to renal injury requires renal angiotensin-converting enzyme. Hypertension. 2015;66:534–42. https://doi.org/10.1161/HYPERTENSIONAHA.115.05320. This study demonstrates that mice lacking renal ACE fail to develop salt sensitivity following treatment with L-NAME due to reduced intrarenal Ang II production and alterations in renal sodium channel activity.
Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–23. https://doi.org/10.1172/JCI65460.
Giani JF, Janjulia T, Kamat N, Seth DM, Blackwell WL, Shah KH, et al. Renal angiotensin-converting enzyme is essential for the hypertension induced by nitric oxide synthesis inhibition. J Am Soc Nephrol. 2014;25:2752–63. https://doi.org/10.1681/ASN.2013091030.
Klimas J, Olvedy M, Ochodnicka-Mackovicova K, Kruzliak P, Cacanyiova S, Kristek F, et al. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med. 2015;19:1965–74. https://doi.org/10.1111/jcmm.12573.
Huskova Z, Kopkan L, Cervenkova L, Dolezelova S, Vanourkova Z, Skaroupkova P, et al. Intrarenal alterations of the angiotensin-converting enzyme type 2/angiotensin 1-7 complex of the renin-angiotensin system do not alter the course of malignant hypertension in Cyp1a1-Ren-2 transgenic rats. Clin Exp Pharmacol Physiol. 2016;43:438–49. https://doi.org/10.1111/1440-1681.12553.
• Kim YG, Lee SH, Kim SY, Lee A, Moon JY, Jeong KH, et al. Sequential activation of the intrarenal renin-angiotensin system in the progression of hypertensive nephropathy in Goldblatt rats. Am J Physiol Renal Physiol. 2016:311, F195–F206. https://doi.org/10.1152/ajprenal.00001.2015. This study describes the temporal changes in ACE and ACE2 expression in the 2K1C model and hypothesizes their relative contribution to the generation and maintenance of hypertension.
•• Chu PL, Gigliotti JC, Cechova S, Bodonyi-Kovacs G, Chan F, Ralph DL, et al. Renal collectrin protects against salt-sensitive hypertension and is downregulated by angiotensin II. J Am Soc Nephrol. 2017;28:1826–37. https://doi.org/10.1681/ASN.2016060675. This study demonstrates through cross-transplantation of collectrin knockout kidneys into wild-type mice that collectrin protects against salt-sensitive hypertension via downregulation of the NHE3 sodium transporter.
Cechova S, Zeng Q, Billaud M, Mutchler S, Rudy CK, Straub AC, et al. Loss of collectrin, an angiotensin-converting enzyme 2 homolog, uncouples endothelial nitric oxide synthase and causes hypertension and vascular dysfunction. Circulation. 2013;128:1770–80. https://doi.org/10.1161/CIRCULATIONAHA.113.003301.
Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–75.
Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614–23.
Shiota A, Yamamoto K, Ohishi M, Tatara Y, Ohnishi M, Maekawa Y, et al. Loss of ACE2 accelerates time-dependent glomerular and tubulointerstitial damage in streptozotocin-induced diabetic mice. Hypertens Res. 2010;33:298–307. https://doi.org/10.1038/hr.2009.231.
Clotet S, Soler MJ, Rebull M, Gimeno J, Gurley SB, Pascual J, et al. Gonadectomy prevents the increase in blood pressure and glomerular injury in angiotensin-converting enzyme 2 knockout diabetic male mice. Effects on renin-angiotensin system. J Hypertens. 2016;34:1752–65. https://doi.org/10.1097/HJH.0000000000001015.
•• Wysocki J, Ye M, Khattab AM, Fogo A, Martin A, David NV, et al. Angiotensin-converting enzyme 2 amplification limited to the circulation does not protect mice from development of diabetic nephropathy. Kidney Int. 2017;91:1336–46. This study finds that increasing systemic ACE2 with recombinant ACE2 does not prevent diabetic changes in GFR, albuminuria, or glomerular histopathology.
Xiao F, Zimpelmann J, Agaybi S, Gurley SB, Puente L, Burns KD. Characterization of angiotensin-converting enzyme 2 ectodomain shedding from mouse proximal tubular cells. PLoS One. 2014;9:e85958. https://doi.org/10.1371/journal.pone.0085958.
Xiao F, Zimpelmann J, Burger D, Kennedy C, Hebert RL, Burns KD. Protein kinase C-delta mediates shedding of angiotensin-converting enzyme 2 from proximal tubular cells. Front Pharmacol. 2016;7:146. https://doi.org/10.3389/fphar.2016.00146.
• Liang Y, Deng H, Bi S, Cui Z, A L, Zheng D, et al. Urinary angiotensin converting enzyme 2 increases in patients with type 2 diabetic mellitus. Kidney Blood Press Res. 2015;40:101–10. https://doi.org/10.1159/000368486. This study demonstrates a correlation between urinary ACE2 to creatinine ratio and various measures metabolic markers of disease including hemoglobin A1C, cholesterol, and blood glucose.
Mariana CP, Ramona PA, Ioana BC, Diana M, Claudia RC, Stefan VD, et al. Urinary angiotensin converting enzyme 2 is strongly related to urinary nephrin in type 2 diabetes patients. Int Urol Nephrol. 2016;48:1491–7. https://doi.org/10.1007/s11255-016-1334-8.
Riera M, Anguiano L, Clotet S, Roca-Ho H, Rebull M, Pascual J, et al. Paricalcitol modulates ACE2 shedding and renal ADAM17 in NOD mice beyond proteinuria. Am J Physiol Renal Physiol. 2016;310:F534–46. https://doi.org/10.1152/ajprenal.00082.2015.
• Lin M, Gao P, Zhao T, He L, Li M, Li Y, et al. Calcitriol regulates angiotensin-converting enzyme and angiotensin converting-enzyme 2 in diabetic kidney disease. Mol Biol Rep. 2016;43:397–406. https://doi.org/10.1007/s11033-016-3971-5. This study demonstrates that calcitriol reduces the renal ACE to ACE2 ratio in diabetic rats with reduction in proteinuria.
Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–38. https://doi.org/10.2337/db09-1218.
Jin HY, Chen LJ, Zhang ZZ, Xu YL, Song B, Xu R, et al. Deletion of angiotensin-converting enzyme 2 exacerbates renal inflammation and injury in apolipoprotein E-deficient mice through modulation of the nephrin and TNF-alpha-TNFRSF1A signaling. J Transl Med. 2015;13:255. https://doi.org/10.1186/s12967-015-0616-8.
• Zheng Y, Tang L, Huang W, Yan R, Ren F, Luo L, et al. Anti-inflammatory effects of Ang-(1-7) in ameliorating HFD-induced renal injury through LDLr-SREBP2-SCAP pathway. PLoS One. 2015;10:e0136187. https://doi.org/10.1371/journal.pone.0136187. This study demonstrates that administration of Ang(1–7) reduces lipid-induced renal injury, inflammation, and lipid deposition through the LDL receptor, SREBP-2, and SCAP pathway.
Velkoska E, Patel SK, Griggs K, Pickering RJ, Tikellis C, Burrell LM. Short-term treatment with diminazene aceturate ameliorates the reduction in kidney ACE2 activity in rats with subtotal nephrectomy. PLoS One. 2015;10:e0118758. https://doi.org/10.1371/journal.pone.0118758.
Anguiano L, Riera M, Pascual J, Valdivielso JM, Barrios C, Betriu A, et al. NEFRONA study. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transplant. 2015;30:1176–85. https://doi.org/10.1093/ndt/gfv025.
Abe M, Maruyama N, Oikawa O, Maruyama T, Okada K, Soma M. Urinary ACE2 is associated with urinary L-FABP and albuminuria in patients with chronic kidney disease. Scand J Clin Lab Invest. 2015;75:421–7. https://doi.org/10.3109/00365513.2015.1054871.
Bae EH, Fang F, Williams VR, Konvalinka A, Zhou X, Patel VB, et al. Murine recombinant angiotensin-converting enzyme 2 attenuates kidney injury in experimental Alport syndrome. Kidney Int. 2017;91:1347–61.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript writing. The authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Culver, Li, and Siragy declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Therapeutic Trials
Rights and permissions
About this article
Cite this article
Culver, S., Li, C. & Siragy, H.M. Intrarenal Angiotensin-Converting Enzyme: the Old and the New. Curr Hypertens Rep 19, 80 (2017). https://doi.org/10.1007/s11906-017-0778-2
Published:
DOI: https://doi.org/10.1007/s11906-017-0778-2