Abstract
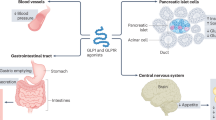
Type 2 diabetes mellitus (T2DM) is a major risk factor for cardiovascular disease. The presence of concomitant hypertension in diabetics is a major driver of excess cardiovascular risk. Glucagon-like peptide-1 receptor agonists (GLP-1a) act on numerous pathways that intersect glycemic, weight, and blood pressure (BP) control. BP-lowering effects have been observed in mouse models of hypertension with a variety of GLP-1a. Acute administration of GLP-1a in humans has been shown to no effects and sometimes increased BP in humans. Chronic administration of GLP-1a, however, reduces clinic systolic BP (≈2 mmHg) at least when evaluated as a secondary end point in glycemia-lowering studies while simultaneously increasing heart rate. BP lowering has not been consistently observed in two recent double-blind controlled clinical trials evaluating ambulatory BP as the primary end point. While a number of mechanisms including vascular, myocardial, renal, and central nervous system pathways have been suggested in animal studies, these mechanistic pathways have not been sufficiently detailed in humans and it is unclear if the same pathways are operational. Further studies need to be conducted to unravel the full spectrum of effects of this drug class. An understanding of their effects on BP may help provide an explanation for the ability of GLP-1a to influence cardiovascular (CV) events in ongoing event-driven CV trials.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52–60.
Nauck M. Incretin therapies—highlighting common features and differences in the modes of action of GLP-1 receptor agonists and DPP-4 inhibitors. Diabetes Obes Metab. 2015; (in press).
Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33(2):187–215.
Lupi R, Del Guerra S, D’Aleo V, Boggi U, Filipponi F, Marchetti P. The direct effects of GLP-1 and GIP, alone or in combination, on human pancreatic islets. Regul Pept. 2010;165(2–3):129–32.
Furman BL. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon. 2012;59(4):464–71.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet Med. 2012;29(10):1260–7.
Glaesner W, Mark Vick A, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26(4):287–96.
Barrington P, Chien JY, Showalter HDH, et al. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(5):426–33.
Christensen M, Knop FK, Vilsbøll T, Holst JJ. Lixisenatide for type 2 diabetes mellitus. Expert Opin Investig Drugs. 2011;20(4):549–57.
Wang B, Zhong J, Lin H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013;15(8):737–49.
Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23(3):334–9.
Gallwitz B, Vaag A, Falahati A, Madsbad S. Adding liraglutide to oral antidiabetic drug therapy: onset of treatment effects over time. Int J Clin Pract. 2010;64(2):267–76.
Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3:e001986.
Katout M, Zhu H, Rutsky J, et al. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am J Hypertens. 2014;27(1):130–9.
Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19(5):567–75. Study in mice that showed that GLP-1R agonists reduce BP by promoting secretion of ANP from the atria and defined a gut-heart axis that regulate BP. The BP effects are GLP-1R dependent and cAMP-Epac dependent.
Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23(7):2939–46. The first paper to show acute BP raising effects of GLP-1 via central sympatehtic mechanisms in rats.
Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006;316(2):852–9.
Isbil-Buyukcoskun N, Gulec G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul Pept. 2004;118(1–2):33–8.
Nyström T, Gonon AT, Sjöholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125(1–3):173–7.
Krisai P, Aeschbacher S, Schoen T, et al. Glucagon-like peptide-1 and blood pressure in young and healthy adults from the general population. Hypertension. 2015; 65(2):306–12.
Bharucha AE, Charkoudian N, Andrews CN, et al. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R874–80.
Devin JK, Pretorius M, Nian H, Yu C, Billings FT, Brown NJ. Dipeptidyl-peptidase 4 inhibition and the vascular effects of glucagon-like peptide-1 and brain natriuretic peptide in the human forearm. J Am Heart Assoc. 2014;3(4): e001075.
Asmar A, Simonsen L, Asmar M, et al. Renal extraction and acute effects of glucagon-like peptide-1 on central and renal hemodynamics in healthy men. Am J Physiol Endocrinol Metab. 2015;308(8):E641–9.
Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38(1):132–9. Liraglutide increases urinary sodium excretion independent of changes in circulating ANP or reduction of SB. Thus suggesting an additional action to that of the cardio-renal GLP-1- ANP axis.
Russell-Jones D, Vaag A, Schmitz O, Sethi B. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009; 52(10):2046–2055.
Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375(9724):1447–56.
Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26(3):268–78.
Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–81.
Buse JB, Sesti G, Schmidt WE, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes 010;33(6):0–3.
Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30(6):1608–10.
Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48(5):592–8.
Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complications. 2014; 28(3):399–405.
Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21(6):1125–35.
Hirata K, Kume S, Araki S, et al. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380(1):44–9.
Laugero KD, Stonehouse AH, Guss S, Landry J, Vu C, Parkes DG. Exenatide improves hypertension in a rat model of the metabolic syndrome. Metab Syndr Relat Disord. 2009;7(4):327–34.
Liu Q, Adams L, Broyde A, Fernandez R, Baron AD, Parkes DG. The exenatide analogue AC3174 attenuates hypertension, insulin resistance, and renal dysfunction in Dahl salt-sensitive rats. Cardiovasc Diabetol. 2010;9:32.
Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43–52.
Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am J Physiol Endocrinol Metab. 1999;277(5):E784–91.
Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes. JAMA. 2015;314(7):687. Large weight loss trial with liraglutide that demonstrates BP (clinic BP) lowering with liraglutide administration. There was also an increase in heart rate noted. A variety of surrogate measures such as hs-CRP, fibrinogen and PAI-1 were favorably modulated.
Ferdinand KC, White WB, Calhoun DA, et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64(4):731–7. First double blind randomized placebo controlled trial of a GLP-1a on ambulatory blood pressure in humans with T2DM. The results showed that chronic daily administration reduces ABP particularly at the higher doses. A possible dose-dependent relationship between GLP-1R agonists and changes in hemodynamic parameters exists, that includes, lowering 24-h SBP (≈3 mmHg), increasing HR (2–4 bpm). These changes occurred as early as 4 weeks and persisted until the end of the study (26 weeks).
Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complications. 2014;28(3):399–405.
Bergenstal RM, Li Y, Porter TKB, Weaver C, Han J. Exenatide once weekly improved glycaemic control, cardiometabolic risk factors and a composite index of an HbA1c < 7%, without weight gain or hypoglycaemia, over 52 weeks. Diabetes Obes Metab. 2013;15(3):264–71.
AstraZeneca; Eli Lilly. Safety and efficacy of exenatide once weekly versus liraglutide in subjects with type 2 diabetes. Clin. [Internet].National Libr. Med.:NCT01029886. http://clinicaltrials.gov/show/NCT01029886.
Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–50.
Rosenstock J, Balas B, Charbonnel B, et al. The fate of taspoglutide, a weekly GLP-1 receptor agonist, versus twice-daily exenatide for type 2 diabetes: the T-emerge 2 trial. Diabetes Care. 2013;36(3):498–504.
Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39–47.
Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159–67.
Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;6736(14):1–9.
Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381(9861):117–24.
Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103–12.
Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists—available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011;13(5):394–407.
Trahair LG, Horowitz M, Hausken T, Feinle-Bisset C, Rayner CK, Jones KL. Effects of exogenous glucagon-like peptide-1 on the blood pressure, heart rate, mesenteric blood flow, and glycemic responses to intraduodenal glucose in healthy older subjects. J Clin Endocrinol Metab. 2014;99(12):E2628–34.
Son JT, Lee E. Comparison of postprandial blood pressure reduction in the elderly by different body position. Geriatr Nurs. 2013;34(4):282–8.
Trahair LG, Horowitz M, Jones KL. Postprandial hypotension: a systematic review. J Am Med Dir Assoc. 2014;15(6):394–409.
Gentilcore D, Bryant B, Wishart JM, Morris HA, Horowitz M, Jones KL. Acarbose attenuates the hypotensive response to sucrose and slows gastric emptying in the elderly. Am J Med. 2005;118(11):1289.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57.
Ravassa S, Zudaire A, Díez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res. 2012;94(2):316–23.
Hällbrink M, Holmqvist T, Olsson M, Ostenson CG, Efendic S, Langel U. Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Galpha(s) and Galpha(i)/Galpha(o) activation. Biochim Biophys Acta. 2001;1546(1):79–86.
Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–65.
Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293(5):E1289–95. GLP-1 enhanced vasodilatation in humans when measured by forearm blood flow. This acetylcholine-mediated vasodilatation was abolished by glyburide. However, glimepiride did not alter the ability of GLP-1 to enhance Ach-mediated vasodilatation.
Nystrom T. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. AJP Endocrinol Metab. 2004;287(6):E1209–15.
Tesauro M, Schinzari F, Adamo A, et al. Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care. 2013;36(3):683–9.
Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept. 2007;141(1–3):120–8.
Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol. 2002;434(3):163–7. http://www.ncbi.nlm.nih.gov/pubmed/11779579.
Rieg T, Gerasimova M, Murray F, et al. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol. 2012;303(7):F963–71.
Gutzwiller J-P, Tschopp S, Bock A, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89(6):3055–61.
Muskiet MHA, Smits MM, Morsink LM, Diamant M. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014;10(2):88–103.
Crajoinas RO, Oricchio FT, Pessoa TD, et al. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol. 2011;301(2):F355–63.
Kocinsky HS, Dynia DW, Wang T, Aronson PS. NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am J Physiol Renal Physiol. 2007;293(1):F212–8.
Skov J, Dejgaard A, Frøkiær J, et al. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab. 2013;98(4):E664–71.
Zhong J, Maiseyeu A, Davis SN, Rajagopalan S. DPP4 in cardiometabolic disease: recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ Res. 2015;116(8):1491–504.
Cabou C, Vachoux C, Campistron G, Drucker DJ, Burcelin R. Brain GLP-1 signaling regulates femoral artery blood flow and insulin sensitivity through hypothalamic PKC. Diabetes. 2011;60(9):2245–56.
Cabou C, Campistron G, Marsollier N, et al. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57(10):2577–87.
Tang-Christensen M, Larsen PJ, Göke R, et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271(4 Pt 2):R848–56.
Skov J, Holst JJ, Gøtze JP, Frøkiær J, Christiansen JS. Glucagon-like peptide-1: effect on pro-atrial natriuretic peptide in healthy males. Endocr Connect. 2014;3(1):11–6.
Li C-J, Yu Q, Yu P, et al. Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol. 2014;13:36.
Zhong J, Maiseyeu A, Rajagopalan S. Lipoprotein effects of incretin analogs and dipeptidyl peptidase 4 inhibitors. Clin Lipidol. 2015; 10(1):103–112.
Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24(1):275–86.
Courrèges J-P, Vilsbøll T, Zdravkovic M, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25(9):1129–31.
Margulies KB. A Randomized trial of liraglutide for high-risk heart failure patients with reduced ejection fraction. Scientific Session. 2015; Oral Presentation.
Shah Z, Pineda C, Kampfrath T, et al. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vasc Pharmacol. 2011;55(1–3):2–9.
Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Drs. Goud, Zhong, Peters, Brook, and Rajagopalan declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Therapeutic Trials
Rights and permissions
About this article
Cite this article
Goud, A., Zhong, J., Peters, M. et al. GLP-1 Agonists and Blood Pressure: A Review of the Evidence. Curr Hypertens Rep 18, 16 (2016). https://doi.org/10.1007/s11906-015-0621-6
Published:
DOI: https://doi.org/10.1007/s11906-015-0621-6