Abstract
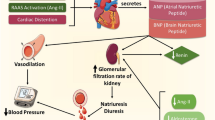
Hypertension is a complex, multifactorial disease, and its development is determined by a combination of genetic susceptibility and environmental factors. Several mechanisms have been implicated in the pathogenesis of hypertension: increased activity of the sympathetic nervous system, overactivation of the renin-angiotensin aldosterone system (RAAS), dysfunction of vascular endothelium, impaired platelet function, thrombogenesis, vascular smooth muscle and cardiac hypertrophy, and altered angiogenesis. MicroRNAs are short, noncoding nucleotides regulating target messenger RNAs at the post-transcriptional level. MicroRNAs are involved in virtually all biologic processes, including cellular proliferation, apoptosis, and differentiation. Thus, microRNA deregulation often results in impaired cellular function and disease development, so microRNAs have potential therapeutic relevance. Many aspects of the development of essential hypertension at the molecular level are still unknown. The elucidation of these processes regulated by microRNAs and the identification of novel microRNA targets in the pathogenesis of hypertension is a highly valuable and exciting strategy that may eventually led to the development of novel treatment approaches for hypertension. This article reviews the potential role of microRNAs in the mechanisms associated with the development and consequences of hypertension and discusses advances in microRNA-based approaches that may be important in treating hypertension.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52.
The world health report 2002—Reducing risks, promoting healthy life. [http://www.who.int/whr/2002/]
Rafiq S, Anand S, Roberts R. Genome-wide association studies of hypertension: have they been fruitful? J Cardiovasc Transl Res. 3(3):189-96
• Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41(6):677–87. This paper reports findings of a genome-wide association study of systolic hypertension and combined meta-analysis of the most promising loci.
Giles TD, Berk BC, Black HR, Cohn JN, Kostis JB, Izzo Jr JL, et al. Expanding the definition and classification of hypertension. J Clin Hypertens (Greenwich). 2005;7(9):505–12.
Nadar SK, Tayebjee MH, Messerli F, Lip GY. Target organ damage in hypertension: pathophysiology and implications for drug therapy. Curr Pharm Des. 2006;12(13):1581–92.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54.
• Bauersachs J, Thum T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res. 2011;109(3):334–47. This article gives an overview of the regulatory role of microRNAs in cardiovascular disease.
Fiedler J, Gupta SK, Thum T. MicroRNA-based therapeutic approaches in the cardiovascular system. Cardiovasc Ther. 2010. doi:10.1111/j.1755-5922.2010.00220.x. Epub ahead of print.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33.
Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5.
Landmesser U, Drexler H. Endothelial function and hypertension. Curr Opin Cardiol. 2007;22(4):316–20.
Felmeden DC, Blann AD, Spencer CG, Beevers DG, Lip GY. A comparison of flow-mediated dilatation and von Willebrand factor as markers of endothelial cell function in health and in hypertension: relationship to cardiovascular risk and effects of treatment: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial. Blood Coagul Fibrinolysis. 2003;14(5):425–31.
Harper RN, Moore MA, Marr MC, Watts LE, Hutchins PM. Arteriolar rarefaction in the conjunctiva of human essential hypertensives. Microvasc Res. 1978;16(3):369–72.
Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101(1):59–68.
Shilo S, Roy S, Khanna S, Sen CK. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(3):471–7.
Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100(8):1164–73.
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71.
Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–84.
Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105(5):1516–21.
Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2(100):ra81.
• Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature 2010;464(7292):1196–200. This article describes the integration of physiological stimulus, growth factor signaling, and angiogenesis by endothelial microRNA-mediated mechanisms.
Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, et al. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One 6(2):e16979.
Fleissner F, Jazbutyte V, Fiedler J, Gupta SK, Yin X, Xu Q, et al. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ Res. 2010;107(1):138–43.
Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, et al. microRNAs in stroke pathogenesis. Curr Mol Med. 2011;11(2):76–92.
Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, et al. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4(11):e7689.
Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120(15):1524–32.
Yang Z, Venardos K, Jones E, Morris BJ, Chin-Dusting J, Kaye DM. Identification of a novel polymorphism in the 3′UTR of the L-arginine transporter gene SLC7A1: contribution to hypertension and endothelial dysfunction. Circulation. 2007;115(10):1269–74.
Yang Z, Kaye DM. Mechanistic insights into the link between a polymorphism of the 3′UTR of the SLC7A1 gene and hypertension. Hum Mutat. 2009;30(3):328–33.
Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124(6):720–30.
Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79(4):581–8.
Hartmann D, Thum T. MicroRNAs and vascular (dys)function. Vascul Pharmacol. 2011 Jul 23 (Epub ahead of print).
Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23(2):171–5.
Bhatt K, Mi QS, Dong Z. microRNAs in kidneys: biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol. 2011;300(3):F602–10.
Liang M, Liu Y, Mladinov D, Cowley Jr AW, Trivedi H, Fang Y, et al. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol. 2009;297(3):F553–8.
•• Lorenzen JM, Haller H, Thum T. MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol. 2011;7(5):286–94. This article provides an overview of microRNAs involved in the pathomechanism in chronic kidney disease.
Naraba H, Iwai N. Assessment of the microRNA system in salt-sensitive hypertension. Hypertens Res. 2005;28(10):819–26.
Liu Y, Taylor NE, Lu L, Usa K, Cowley Jr AW, Ferreri NR, et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55(4):974–82.
Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, et al. Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am J Hypertens. 2010;23(1):78–84.
Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52(4):639–72.
Martin MM, Lee EJ, Buckenberger JA, Schmittgen TD, Elton TS. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J Biol Chem. 2006;281(27):18277–84.
Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, et al. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3′ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet. 2007;81(2):405–13.
Mottl AK, Shoham DA, North KE. Angiotensin II type 1 receptor polymorphisms and susceptibility to hypertension: a HuGE review. Genet Med. 2008;10(8):560–74.
Yael Nossent A, Hansen JL, Doggen C, Quax PH, Sheikh SP, Rosendaal FR. SNPs in microRNA binding sites in 3′-UTRs of RAAS genes influence arterial blood pressure and risk of myocardial infarction. Am J Hypertens. 2011;24(9):999–1006.
Wang K, Long B, Zhou J, Li PF. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem. 2010;285(16):11903–12.
Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, et al. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci USA. 1998;95(16):9424–9.
Söber S, Laan M, Annilo T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem Biophys Res Commun. 2010;391(1):727–32.
Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801.
Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78(2):274–85.
Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 2010;30(6):1118–26.
Albinsson S, Skoura A, Yu J, DiLorenzo A, Fernandez-Hernando C, Offermanns S, et al. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PLoS One. 2011;6(4):e18869.
Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119(9):2634–47.
Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23(18):2166–78.
Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16(12):1590–8.
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–10.
Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105(2):158–66.
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–4.
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100(11):1579–88.
Jazbutyte V, Thum T. MicroRNA-21: from cancer to cardiovascular disease. Curr Drug Targets. 2010;11(8):926–35.
Kotlo KU, Hesabi B, Danziger RS. Implication of microRNAs in atrial natriuretic peptide- and nitric oxide signaling in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2011;301(4):C929–37.
Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104(4):476–87.
Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284(6):3728–38.
• Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124(2):175–84. This is the first report of a circulating miRNA profile in hypertensive patients. The authors also demonstrate a novel link between HCMV infection and essential hypertension.
Wessely R, Hengst L, Jaschke B, Wegener F, Richter T, Lupetti R, et al. A central role of interferon regulatory factor-1 for the limitation of neointimal hyperplasia. Hum Mol Genet. 2003;12(2):177–87.
O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, et al. Heritability and genome-wide linkage in US and Australian twins identify novel genomic regions controlling chromogranin A: implications for secretion and blood pressure. Circulation. 2008;118(3):247–57.
Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–9.
Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28(4):341–7.
• Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-Mediated Upregulation of miR-21 Promotes Renal Fibrosis. J Am Soc Nephrol. 2011;22(9):1668–81. This paper describes the role of miR-21 in TGF-β-induced renal fibrosis as a downstream target of Smad-3 and demonstrates the potential of knocking down miR-21 in reducing fibrosis development.
Liu G, Friggeri A, Yang YP, Milosevic J, Ding QA, Thannickal VJ, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207(8):1589–97.
• Thum T, Chau N, Bhat B, Gupta SK, Linsley PS, Bauersachs J, et al. Comparison of different miR-21 inhibitor chemistries in a cardiac disease model. J Clin Invest. 2011;121(2):461–2; author reply 462–463. In this paper, the authors demonstrate the differences in biologic effects of microRNA inhibitor chemistries.
Kim JH, Yeom JH, Ko JJ, Han MS, Lee K, Na SY, et al. Effective delivery of anti-miRNA DNA oligonucleotides by functionalized gold nanoparticles. J Biotechnol. 2011;155(3):287–92.
Santaris Pharma: Multiple Ascending Dose Study of Miravirsen in Treatment-Naïve Chronic Hepatitis C Subjects. In: Clinical Trials.gov. Bethesda (MD): National Library of Medicine (US). [cited 2011 Oct 04]. Available from: http://clinicaltrials.gov/ct2/show/NCT01200420. NLM Identifier: NCT01200420.
Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4(9):721–6.
Ebert MS, Sharp PA. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20(19):R858–61.
Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70(18):7027–30.
Disclosure
The authors disclose support from the IFB-Tx (BMBF 01EO0802; T.T.), DFG TH 903/10-1 (T.T.) and FP7 ERG 294278 (S.B. and T.T.). T.T. has filed patents in the field of cardiovascular microRNA diagnostics and therapeutics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bátkai, S., Thum, T. MicroRNAs in Hypertension: Mechanisms and Therapeutic Targets. Curr Hypertens Rep 14, 79–87 (2012). https://doi.org/10.1007/s11906-011-0235-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-011-0235-6