Abstract
Purpose of Review
Despite highly effective biomedical HIV pre-exposure prophylaxis (PrEP) options, suboptimal PrEP uptake impedes progress towards ending the epidemic in the United States of America (USA). Implementation science bridges what we know works in controlled clinical trial settings to the context and environment in which efficacious tools are intended to be deployed. In this review, we focus on strategies that target PrEP use barriers at the system or structural level, exploring the implications and opportunities in the context of the fragmented USA healthcare system.
Recent Findings
Task shifting could increase PrEP prescribers, but effectiveness evidence is scarce in the USA, and generally focused in urban settings. Integration of PrEP within existing healthcare infrastructure concentrates related resources, but demonstration projects rarely present the resource implications of redirecting staff. Changing the site of service via expanded telehealth could improve access to more rural populations, though internet connectivity, technology access, and challenges associated with determining biomedical eligibility remain logistical barriers for some of the highest burden communities in the USA. Finally, a tailored care navigation and coordination approach has emerged as a highly effective component of PrEP service provision, attempting to directly modify the system-level determinants of PrEP use experienced by the individual.
Summary
We highlight recent advances and evidence surrounding task shifting, integration, service delivery, and tailoring. With the exception of tailored care navigation, evidence is mixed, and the downstream impact and sustainability of task shifting and care integration require further attention. To maximize PrEP outcomes, research will need to continue to examine the interplay between individuals, clinics, and the healthcare system and associated policies within which they operate.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Smith DK, Sullivan PS, Cadwell B, et al. Evidence of an association of increases in pre-exposure prophylaxis coverage with decreases in human immunodeficiency virus diagnosis rates in the United States, 2012–2016. Clin Infect Dis. 2020;71(12):3144–51.
Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. Core indicators for monitoring the Ending the HIV Epidemic initiative (preliminary data): National HIV Surveillance System data reported through June 2021; and preexposure prophylaxis (PrEP) data reported through March 2021. HIV Surveillance Data Tables 2021;2(No. 4). Table 3a. Number of Persons Prescribed PrEP, Number of Persons with PrEP Indications, and PrEP Coverage during January 2019 through March 2021, among Persons Aged >= 16 Years, by Selected Characteristics - United States (Preliminary). Published October 2021. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/vol-2-no-4/index.html. Accessed 4 May 2023.
Smith DK, Van Handel M, Wolitski RJ, et al. Vital signs: Estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition–United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(46):1291–5.
Siegler AJ, Bratcher A, Weiss KM. Geographic access to preexposure prophylaxis clinics among men who have sex with men in the United States. Am J Public Health. 2019;109(9):1216–23.
Sharpe JD, Guest JL, Siegler AJ, Sanchez TH, Sullivan PS. The spatiotemporal distribution of pre-exposure prophylaxis accessibility in the United States, 2016–2020. Ann Epidemiol. Published online September 24, 2021. https://doi.org/10.1016/j.annepidem.2021.09.006
Karen W . Hoover, Weiming Zhu, Jeffrey Wiener, Ya-Lin A. Huang. Trends in truvada and descovy prescriptions for PrEP in the United States, 2014–2020. https://www.natap.org/2021/CROI/croi_201.htm. Accessed 11 Oct 2021.
HIV in the Southern United States. Published September 2019. https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-in-the-south-issue-brief.pdf. Accessed 27 Sep 2021.
South - AIDSVu. Published August 5, 2019. https://aidsvu.org/local-data/united-states/south/. Accessed 21 Sep 2021.
Hosek SG, Rudy B, Landovitz R, et al. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74(1):21–9.
Hosek SG, Landovitz RJ, Kapogiannis B, et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr. 2017;171(11):1063–71.
Rolle CP, Rosenberg ES, Luisi N, et al. Willingness to use pre-exposure prophylaxis among Black and White men who have sex with men in Atlanta, Georgia. Int J STD AIDS. 2017;28(9):849–57.
Huang YLA, Zhu W, Smith DK, Harris N, Hoover KW. HIV Preexposure prophylaxis, by race and ethnicity - United States, 2014–2016. MMWR Morb Mortal Wkly Rep. 2018;67(41):1147–50.
Bush S, Magnuson D, Rawlings MK, Hawkins T, McCallister S, Mera Giler R. Racial Characteristics of FTC/TDF for pre-exposure prophylaxis (PrEP) users in the USA. https://www.natap.org/2016/HIV/062216_02.htm. Accessed 27 Sep 2021.
Hubach RD, Currin JM, Sanders CA, et al. Barriers to access and adoption of pre-exposure prophylaxis for the prevention of HIV among men who have sex with men (MSM) in a relatively rural state. AIDS Educ Prev. 2017;29(4):315–29.
Sharpe JD, Sanchez TH, Siegler AJ, Guest JL, Sullivan PS. Association between the geographic accessibility of PrEP and PrEP use among MSM in nonurban areas. J Rural Health. 2022;38(4):948–59.
Owens C, Hubach RD, Williams D, et al. Facilitators and barriers of pre-exposure prophylaxis (PrEP) uptake among rural men who have sex with men living in the midwestern U.S. Arch Sex Behav. 2020;49(6):2179–91.
Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28(12):841–9.
Mayer KH, Agwu A, Malebranche D. Barriers to the wider use of pre-exposure prophylaxis in the United States: a narrative review. Adv Ther. 2020;37(5):1778–811.
Antonini M, da Silva IE, Elias HC, Gerin L, Oliveira AC, Reis RK. Barriers to pre-exposure prophylaxis (PrEP) use for HIV: an integrative review. Rev Bras Enferm. 2023;76(3): e20210963.
• Hillis A, Germain J, Hope V, McVeigh J, Van Hout MC. Pre-exposure prophylaxis (PrEP) for HIV prevention among men who have sex with men (MSM): a scoping review on PrEP service delivery and programming. AIDS Behav. 2020;24(11):3056–70 This review of literature published from 2008 to 2019 is important as it illustrates the myriad of pathways to PrEP that MSM are compelled to pursue in the current health system/PrEP services environments and underscores the importance of tailored approaches to PrEP delivery for sub-populations within MSM communities.
Sullivan PS, Mena L, Elopre L, Siegler AJ. Implementation strategies to increase PrEP uptake in the south. Curr HIV/AIDS Rep. 2019;16(4):259–69.
Complete List of PrEP Best Practices. Published January 2, 2024. https://www.cdc.gov/hiv/research/interventionresearch/compendium/prep/complete-list.html. Accessed 10 Feb 2024.
• Velloza J, Roche S, Concepcion T, Ortblad KF. Advancing considerations of context in the evaluation and implementation of evidence-based biomedical HIV prevention interventions: a review of recent research. Curr Opin HIV AIDS. 2023;18(1):1–11. This review focused on recent findings from daily oral PrEP and the dapivirine vaginal ring (DPV) prevention technologies to illustrate and emphasize how different biomedical prevention technologies can be better (or more poorly) suited to particular population, systems, cultural and historical contexts—encouraging a more nuanced approach to implementation decision-making rather than based solely on clinical trial results.
Pinto RM, Berringer KR, Melendez R, Mmeje O. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22(11):3681–91.
Sivashanker Karthik, Duong Tam, Resnick Andrew, Eappen Sunil. Health care equity: from fragmentation to transformation. Catalyst non-issue content. 1(5). https://doi.org/10.1056/CAT.20.0414
Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
Elhauge E, ed. Why we should care about health care fragmentation and how to fix it. In: The Fragmentation of U.S. Health Care: Causes and Solutions. Oxford; :1–20.
Chehal PK, Selvin E, DeVoe JE, Mangione CM, Ali MK. Diabetes and the fragmented state Of USA health care and policy. Health Aff. 2022;41(7):939–46.
Liu AY, Scott HM, Buchbinder SP. New USPSTF guidelines for HIV preexposure prophylaxis: will more choices lead to greater impact? JAMA. 2023;330(8):699–701.
Prevention of Acquisition of HIV: Preexposure prophylaxis. Published August 22, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed 5 Nov 2023.
Sobel L, Ranji U, Pestaina K, Dawson L, Cubanski J. [No title]. https://www.kff.org/womens-health-policy/issue-brief/explaining-litigation-challenging-the-acas-preventive-services-requirements-braidwood-management-inc-v-becerra/. Accessed 27 Sep 2023.
•• Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608. This landmark clinical trial established the superiority of long-acting injectable cabotegravir (CAB-LA) over daily oral PrEP for HIV prevention among MSM and transgender women. The trial was halted early due to efficacy. It is also selected here as noteworthy to mark the failure of the USA health system to date to scale up this highly effective prevention technology.
Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. Published online April 1, 2022. https://doi.org/10.1016/S0140-6736(22)00538-4
Patel RR, MPH, Khan T, et al. From prescription to patient: the lifecycle of cabotegravir for PrEP. https://www.idsociety.org/science-speaks-blog/2023/from-prescription-to-patient-the-lifecycle-of-cabotegravir-for-prep/#/+/0/publishedDate_na_dt/desc/. Accessed 5 Nov 2023.
Cabotegravir GLAI. Cost of goods sold (COGS) analysis: https://www.clintonhealthaccess.org/wp-content/uploads/2022/10/Generic-CAB-LA-COGS-Analysis.pdf. Accessed 5 Nov 2023.
Pepperrell T, Cross S, Hill A. Cabotegravir-global access to long-acting pre-exposure prophylaxis for HIV. Open Forum Infect Dis. 2023;10(1):ofac673.
Sharma I, Hill A. Global HIV incidence analysis and implications for affordability using CAB-LA versus continuous and event-driven oral PrEP. Clin Infect Dis. Published online September 4, 2023. https://doi.org/10.1093/cid/ciad537
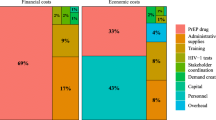
• Furukawa NW, Zhu W, Huang YLA, Shrestha RK, Hoover KW. National trends in drug payments for HIV preexposure prophylaxis in the United States, 2014 to 2018: A retrospective cohort study. Ann Intern Med. 2020;173(10):799–805. This robust analysis of > 90% of USA retail pharmacy prescriptions for PrEP (2014–2018) provides detailed information about overall estimated out-of-pocket costs and differences in out-of-pocket costs by health insurance type and demographic characteristics. The analysis also examines temporal trends in cost and estimates total costs to the healthcare system for PrEP medication payments.
•• Srikanth K, Killelea A, Strumpf A, Corbin-Gutierrez E, Horn T, McManus KA. Associated costs are a barrier to HIV preexposure prophylaxis access in the United States. Am J Public Health. 2022;112(6):834–8. This recent editorial provides a concise synthesis of the individual and systems-level cost challenges that are inhibiting broader PrEP expansion in the USA. A helpful summary table is included illustrating the fractured nature of the financial assistance programs for PrEP and what they do and do not cover in terms of medications, lab tests, and associated healthcare services.
Farmer EK, Koren DE, Cha A, Grossman K, Cates DW. The pharmacist’s expanding role in HIV pre-exposure prophylaxis. AIDS Patient Care STDS. 2019;33(5):207–13.
Myers JE, Farhat D, Guzman A, Arya V. Pharmacists in HIV prevention: an untapped potential. Am J Public Health. 2019;109(6):859–61.
Lopez MI, Grant RM, Dong BJ. Community pharmacy delivered PrEP to STOP HIV transmission: an opportunity NOT to miss! J Am Pharm Assoc. 2020;60(4):e18–24.
Rousseau E, Julies RF, Madubela N, Kassim S. Novel platforms for biomedical HIV prevention delivery to key populations - community mobile clinics, peer-supported, pharmacy-led PrEP delivery, and the use of telemedicine. Curr HIV/AIDS Rep. 2021;18(6):500–7.
• Zhao A, Dangerfield DT 2nd, Nunn A, et al. Pharmacy-based interventions to increase use of HIV pre-exposure prophylaxis in the United States: A Scoping Review. AIDS Behav. 2022;26(5):1377–92. This scoping review included peer-reviewed articles published between 2012 and 2021 and conducted in the USA that described the potential impact of pharmacy-affiliated PrEP care on PrEP uptake and/or use. Ultimately including 33 empirical studies and 16 reviews, the topics focused on pharmacist knowledge/perceptions and pharmacy-based PrEP implementation. The authors note a paucity of data regarding pharmacist knowledge or general PrEP familiarity outside of high-burden urban settings, highlighting an increased need for PrEP education among pharmacists. Nearly all intervention studies were observational and did not include a control group for comparison and few studies directly addressed the structural barriers that could impede PrEP uptake or persistence.
Kennedy CE, Yeh PT, Atkins K, Ferguson L, Baggaley R, Narasimhan M. PrEP distribution in pharmacies: a systematic review. BMJ Open. 2022;12(2): e054121.
Khosropour CM, Backus KV, Means AR, et al. A pharmacist-led, same-day, HIV pre-exposure prophylaxis initiation program to increase PrEP uptake and decrease time to PrEP initiation. AIDS Patient Care STDS. 2020;34(1):1–6.
Khosropour CM, Riley T, Healy E, et al. Persistence in a pharmacist-led, same-day PrEP program in Mississippi: a mixed-methods study. BMC Public Health. 2023;23(1):1130.
American Medical Association. Don’t expand scope of practice for already overworked pharmacists. American Medical Association. Published May 16, 2023. https://www.ama-assn.org/practice-management/scope-practice/don-t-expand-scope-practice-already-overworked-pharmacists. Accessed 27 Sep 2023.
Academy Warns Against Expanding Pharmacists’ Scope. Published December 14, 2022. https://www.aafp.org/news/government-medicine/pharm-scope-expansion-warning.html. Accessed 27 Sep 2023.
HOUSE BILL 182 – Idaho State Legislature. https://legislature.idaho.gov/sessioninfo/2019/legislation/h0182/. Accessed 27 Sep 2023.
Tannenbaum C, Tsuyuki RT. The expanding scope of pharmacists’ practice: implications for physicians. CMAJ. 2013;185(14):1228–32.
Fisher HH, Hoyte T, Purcell DW, et al. Health department HIV prevention programs that support the national HIV/AIDS strategy: the enhanced comprehensive HIV prevention planning project, 2010–2013. Public Health Rep. 2016;131(1):185–94.
Tarek Mikati, Kelly Jamison, Demetre C. Daskalakis. Immediate PrEP Initiation at New York City Sexual Health Clinics. In: ; 2019. https://www.croiconference.org/abstract/immediate-prep-initiation-new-york-city-sexual-health-clinics/. Accessed 3 Oct 2021.
Chan PA, Glynn TR, Oldenburg CE, et al. Implementation of preexposure prophylaxis for human immunodeficiency virus prevention among men who have sex with men at a New England sexually transmitted diseases clinic. Sex Transm Dis. 2016;43(11):717–23.
Kamis KF, Marx GE, Scott KA, et al. Same-day HIV pre-exposure prophylaxis (PrEP) initiation during drop-in sexually transmitted diseases clinic appointments is a highly acceptable, feasible, and safe model that engages individuals at risk for HIV into PrEP care. Open Forum Infect Dis. 2019;6(7):ofz310.
Reynolds HW, Sutherland EG. A systematic approach to the planning, implementation, monitoring, and evaluation of integrated health services. BMC Health Serv Res. 2013;13:168.
Shigayeva A, Atun R, McKee M, Coker R. Health systems, communicable diseases and integration. Health Policy Plan. 2010;25 Suppl 1(Suppl 1):i4-20.
Bauermeister JA, Golinkoff JM, Horvath KJ, Hightow-Weidman LB, Sullivan PS, Stephenson R. A multilevel tailored web app–based intervention for linking young men who have sex with men to quality care (get connected): protocol for a randomized controlled trial. JMIR Res Protoc. 2018;7(8): e10444.
Bhatia R, Modali L, Lowther M, et al. Outcomes of preexposure prophylaxis referrals from public STI clinics and implications for the preexposure prophylaxis continuum. Sex Transm Dis. 2018;45(1):50–5.
Kwakwa HA, Bessias S, Sturgis D, et al. Engaging United States black communities in HIV pre-exposure prophylaxis: analysis of a PrEP engagement cascade. J Natl Med Assoc. 2018;110(5):480–5.
• Casey E, Kaplan-Lewis E, Gala K, Lakew R. Successful integration of HIV PrEP in primary care and women’s health clinical practice: a model for implementation. Viruses. 2023;15(6). https://doi.org/10.3390/v15061365. In this prospective cohort study, investigators enrolled health care providers in primary care and women’s health clinics in New York City in a three-prong intervention: a virtual PrEP curriculum for all providers, provision of site-specific list of PrEP-eligible patients seen in a clinic in the prior 6 months (based on incident STI), and, for one hospital, embedding a dedicated staffer within women’s health and primary care clinics to support PrEP implementation needs specific to those clinic-specific resources. Investigators noted an increase in the proportion prescribing PrEP before (12; 11.5%) versus after (51; 49%) and an increase in a total number of patients on PrEP (19 to 128). Using education, audit-and-feedback, and embedded champions to address PrEP implementation barriers, this work offers a signal of success, but is limited by a lack of control population or clinics, no data on actual use or persistent use PrEP (just the prescription), and unclear sustainability or necessary resources.
Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure PROPHYLAXIS for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176(1):75–84.
Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on telehealth and telemedicine as applied to the practice of infectious diseases. Clin Infect Dis. 2019;68(9):1437–43.
Stekler JD, McMahan V, Ballinger L, et al. HIV Pre-exposure prophylaxis prescribing through telehealth. J Acquir Immune Defic Syndr. 2018;77(5):e40–2.
Touger R, Wood BR. A review of telehealth innovations for HIV pre-exposure prophylaxis (PrEP). Curr HIV/AIDS Rep. 2019;16(1):113–9.
Siegler AJ, Mayer KH, Liu AY, et al. Developing and assessing the feasibility of a home-based preexposure prophylaxis monitoring and support program. Clin Infect Dis. 2019;68(3):501–4.
Esmaeili ED, Azizi H, Dastgiri S, Kalankesh LR. Does telehealth affect the adherence to ART among patients with HIV? A systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):169.
• Salgado S, Felzien G, Brumbeloe J. Georgia leverages telehealth to expand HIV care management in underserved areas. Am J Prev Med. 2021;61(5 Suppl 1):S55–9. This publication showcases a highly successful, state-level implementation initiative of telehealth services for HIV care. HIV care access was greatly expanded across the state, and patients who had telehealth visits were virally suppressed (91.4%) and comparable to the broader patient population (Georgia Ryan White HIV/AIDS Program Part B) overall.
Greenwell K, Fugit R, Nicholson L, Wright J. A Retrospective comparison of HIV pre-exposure prophylaxis (PrEP) outcomes between a pharmacist-led telehealth clinic and in-person clinic in a veteran population. AIDS Behav. Published online May 29, 2023:1–9.
Patel P, Kerzner M, Reed JB, Sullivan PS, El-Sadr WM. Public health implications of adapting HIV pre-exposure prophylaxis programs for virtual service delivery in the context of the COVID-19 pandemic: Systematic review. JMIR Public Health Surveill. 2022;8(6): e37479.
• Higgins DM, Riba A, Alderton L, et al. Evaluation of the impact and outcomes of a rapid transition to telehealth PrEP delivery at a sexual health clinic during the COVID-19 pandemic. Sex Transm Dis. Published online October 9, 2023. https://doi.org/10.1097/OLQ.0000000000001872. This retrospective analysis of clinic records at a Denver Sexual Health Clinic suggests sustained engagement in PrEP services with the COVID-19-driven transition to telePrEP visits. PrEP visit volume remained largely stable in the “post-COVID-19” period without a significant shift in the demographics of PrEP initiators among the measures collected. The authors note a drop off in the proportion of clients with documented prescription dispensing in the “post-COVID-19” period but observed similar 3-month PrEP retention rates in both pre/post-COVID-19 periods and comparing persons with in-clinic and telePrEP only visits. Importantly, all PrEP clients engaged in care at this clinic receive PrEP navigation services—an increasingly important evidence-based intervention for PrEP persistence.
Labisi T, Regan N, Davis P, Fadul N. HIV care meets telehealth: a review of successes, disparities, and unresolved challenges. Curr HIV/AIDS Rep. 2022;19(5):446–53.
Grove M, Brown LL, Knudsen HK, Martin EG, Garner BR. Employing telehealth within HIV care: advantages, challenges, and recommendations. AIDS. 2021;35(8):1328–30.
•• Siegler A, Sullivan P. The PrEP laboratory service gap: applying implementation science strategies to bring PrEP coverage to scale in the United States. J Law Med Ethics. 2022;50(S1):40–6. This article highlights one critical systems-level component inhibiting the broader expansion of PrEP services within the USA—the lack of comprehensive coverage of lab services required to initiate and maintain PrEP care. Using an Implementation Science framework, the authors propose core elements of a national laboratory services program that would be needed to support a national PrEP program initiative, including, among other things, simplifying billing, changing patient fees, providing centralized technology assistance, and expanding telehealth service components.
Roy M, Bolton Moore C, Sikazwe I, Holmes CB. A review of differentiated service delivery for HIV treatment: effectiveness, mechanisms, targeting, and scale. Curr HIV/AIDS Rep. 2019;16(4):324–34.
Luke DA, Calhoun A, Robichaux CB, Elliott MB, Moreland-Russell S. The program sustainability assessment tool: a new instrument for public health programs. Prev Chronic Dis. 2014;11: 130184.
Howren MB, Francis SL, Polgreen LA, Shafer C, Hoth A, Ohl ME. Predictors of HIV preexposure prophylaxis initiation among public health clients in rural and small urban areas in Iowa. Public Health Rep. 2021;136(2):172–82.
Frank L, Starzyk E, Hoxworth T, et al. HIV PrEP implementation: a multi-level systems approach. Eval Program Plann. Published online May 30, 2021:101966.
Pathela P, Jamison K, Blank S, Daskalakis D, Hedberg T, Borges C. The HIV pre-exposure prophylaxis (PrEP) cascade at NYC sexual health clinics: navigation is the key to uptake. J Acquir Immune Defic Syndr. 2020;83(4):357–64.
•• Kimball AA, Zhu W, Tanner MR, et al. The effect of navigation on linkage to a PrEP provider among PrEP-eligible men who have sex with men in a U.S. Demonstration project. AIDS Behav. Published online November 22, 2022. https://doi.org/10.1007/s10461-022-03931-y. The THRIVE national CDC demonstration project (n = 9538 PrEP-eligible MSM) is noteworthy as it had a wide range across study sites in the proportion of eligible screened participants linked to PrEP services (10.7 to 95.9%, mean 53.8%), illustrated the “drop-off” along the cascade with substantially fewer being prescribed PrEP (37.2%), and demonstrated the impact of PrEP navigation (48.5% of MSM who used navigation were linked to PrEP as compared to 2.8% being linked among those who did not use navigation).
Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321(9):844–5.
Gamarel KE, King WM, Operario D. Behavioral and social interventions to promote optimal HIV prevention and care continua outcomes in the United States. Curr Opin HIV AIDS. 2022;17(2):65–71.
Rousseau E, Bekker LG, Julies RF, et al. A community-based mobile clinic model delivering PrEP for HIV prevention to adolescent girls and young women in Cape Town, South Africa. BMC Health Serv Res. 2021;21(1):888.
Roche SD, Odoyo J, Irungu E, et al. A one-stop shop model for improved efficiency of pre-exposure prophylaxis delivery in public clinics in western Kenya: a mixed methods implementation science study. J Int AIDS Soc. 2021;24(12): e25845.
Wouters E, Van Damme W, van Rensburg D, Masquillier C, Meulemans H. Impact of community-based support services on antiretroviral treatment programme delivery and outcomes in resource-limited countries: a synthetic review. BMC Health Serv Res. 2012;12(1):194.
Naburi H, Ekström AM, Mujinja P, et al. The potential of task-shifting in scaling up services for prevention of mother-to-child transmission of HIV: a time and motion study in Dar es Salaam, Tanzania. Hum Resour Health. 2017;15(1):35.
Irungu E, Khoza N, Velloza J. Multi-level interventions to promote oral pre-exposure prophylaxis use among adolescent girls and young women: a review of recent research. Curr HIV/AIDS Rep. 2021;18(6):490–9.
O’Malley G, Barnabee G, Mugwanya K. Scaling-up PrEP delivery in Sub-Saharan Africa: what can we learn from the scale-up of ART? Curr HIV/AIDS Rep. 2019;16(2):141–50.
Belay YA, Yitayal M, Atnafu A, Taye FA. Barriers and facilitators to the implementation and scale up of differentiated service delivery models for HIV treatment in Africa: a scoping review. BMC Health Serv Res. 2022;22(1):1431.
Grimsrud A, Wilkinson L, Delany-Moretlwe S, et al. The importance of the “how”: the case for differentiated service delivery of long-acting and extended delivery regimens for HIV prevention and treatment. J Int AIDS Soc. 2023;26 Suppl 2(Suppl 2):e26095.
Guilamo-Ramos V, Thimm-Kaiser M, Benzekri A, Futterman D. Shifting the paradigm in HIV prevention and treatment service delivery toward differentiated care for youth. NAM Perspect. Published online March 25, 2019. https://doi.org/10.31478/201903a
Funding
Dr. Rutstein was supported by Doris Duke Charitable Foundation grant #2015213 and University of North Carolina at Chapel Hill Center for AIDS Research (P30AI50410). Drs. Rutstein and Muessig were supported by R61AI174285.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rutstein, S.E., Muessig, K.E. Leveling Up PrEP: Implementation Strategies at System and Structural Levels to Expand PrEP Use in the United States. Curr HIV/AIDS Rep 21, 52–61 (2024). https://doi.org/10.1007/s11904-024-00697-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-024-00697-x