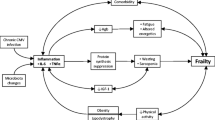
Abstract
Purpose of Review
An increasing body of evidence indicates that persons living with HIV (PLWH) display dysfunctional immunometabolism. Here, we provide an updated review of this topic and its relationship to HIV-associated immune stimuli and age-related disease.
Recent Findings
HIV infection alters immunometabolism by increasing reliance on aerobic glycolysis for energy and productive infection and repurposing oxidative phosphorylation machinery for immune cell proliferation and survival. Recent studies in PLWH with diabetes mellitus or cardiovascular disease have identified an association with elevated T cell and monocyte glucose metabolism, respectively. Immunometabolic dysfunction has also been observed in PLWH in frailty and additional studies suggest a role for immunometabolism in non-AIDS defining cancers and neurocognitive disease. There is a plethora of HIV-associated immune stimuli that could drive immunometabolic dysfunction and age-related disease in PLWH, but studies directly examining their relationship are lacking.
Summary
Immunometabolic dysfunction is characteristic of HIV infection and is a potential link between HIV-associated stimuli and age-related comorbidities.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4 + T cell gains in human immunodeficiency virus – infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. https://doi.org/10.1086/374786.
Vinikoor MJ, Cope A, Gay CL, Ferrari G, McGee KS, Kuruc JD, et al. Antiretroviral therapy initiated during acute HIV infection fails to prevent persistent T-cell activation. J Acquir Immune Defic Syndr. 2013;62:505–8. https://doi.org/10.1097/QAI.0b013e318285cd33.
Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, et al. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. 2017;64:124–31. https://doi.org/10.1093/cid/ciw683.
Hearps AC, Maisa A, Cheng W-J, Angelovich TA, Lichtfuss GF, Palmer CS, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843–53. https://doi.org/10.1097/QAD.0b013e328351f756.
Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–6. https://doi.org/10.1093/cid/cir627.
The Antiretroviral Therapy Cohort Collaboration Study Group. Causes of death in HIV-1–infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–96. https://doi.org/10.1086/652283.
Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non – AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210:1248–59. https://doi.org/10.1093/infdis/jiu254.
Duffau P, Wittkop L, Lazaro E, Le Marec F, Cognet C, Blanco P, et al. Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS. 2015;29:2099–108. https://doi.org/10.1097/QAD.0000000000000807.
Krishnan S, Wilson EMP, Sheikh V, Rupert A, Mendoza D, Yang J, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis. 2014;209:931–9. https://doi.org/10.1093/infdis/jit581.
Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–43. https://doi.org/10.1016/j.immuni.2013.04.005.
Le Zhang L, Zhang ZN, Wu X, Jiang YJ, Fu YJ, Shang H. Transcriptomic meta-analysis identifies gene expression characteristics in various samples of HIV-infected patients with nonprogressive disease. J Transl Med. 2017;15:1–10. https://doi.org/10.1186/s12967-017-1294-5.
Wu JQ, Dwyer DE, Dyer WB, Yang YH, Wang B, Saksena NK. Genome-wide analysis of primary CD4+ and CD8+ T cell transcriptomes shows evidence for a network of enriched pathways associated with HIV disease. Retrovirology. 2011;8:18. https://doi.org/10.1186/1742-4690-8-18.
•• Tarancon-Diez L, Rodríguez-Gallego E, Rull A, Peraire J, Viladés C, Portilla I, et al. Immunometabolism is a key factor for the persistent spontaneous elite control of HIV-1 infection. EBioMedicine. 2019;42:86–96. https://doi.org/10.1016/j.ebiom.2019.03.004. This study describes the metabolomic profile of PLWH who lose virological control, which is characterized by aerobic glycolysis, dysfunctional mitochondria and oxidative stress. Importantly, decreased functionality of CD8+ T cells was found to be associated with dysfunctional mitochondria and oxidative stress.
• Kavanagh Williamson M, Coombes N, Juszczak F, Athanasopoulos M, Khan M, Eykyn T, et al. Upregulation of glucose uptake and hexokinase activity of primary human CD4+ T cells in response to infection with HIV-1. Viruses. 2018;10:114. https://doi.org/10.3390/v10030114. This study provides a comprehensive assessment of glucose transporter mRNA and protein expression in resting CD4+ T cells and CD3/CD28-stimulated uninfected and HIV-infected CD4+ T cells.
Loisel-Meyer S, Swainson L, Craveiro M, Oburoglu L, Mongellaz C, Costa C, et al. Glut1-mediated glucose transport regulates HIV infection. Proc Natl Acad Sci USA. 2012;109:2549–54. https://doi.org/10.1073/pnas.1121427109.
• Valle-Casuso JC, Angin M, Volant S, Passaes C, Monceaux V, Mikhailova A, et al. Cellular metabolism is a major determinant of HIV-1 reservoir seeding in CD4+ T cells and offers an opportunity to tackle infection. Cell Metab. 2019;29:611–626.e5. https://doi.org/10.1016/j.cmet.2018.11.015. This study demonstrates that HIV preferentially targets the most metabolically active CD4+ T cells, the effector memory subset, and that the metabolic program of the cell dictates the outcome of HIV infectionin vitro.
Hegedus A, Kavanagh Williamson M, Huthoff H. HIV-1 pathogenicity and virion production are dependent on the metabolic phenotype of activated CD4+ T cells. Retrovirology. 2014;11:98. https://doi.org/10.1186/s12977-014-0098-4.
• Hegedus A, Kavanagh Williamson M, Khan MB, Dias Zeidler J, Da Poian AT, El-Bacha T, et al. Evidence for altered glutamine metabolism in human immunodeficiency virus type 1 infected primary human CD4+ T cells. AIDS Res Hum Retroviruses. 2017;33:1236–47. https://doi.org/10.1089/aid.2017.0165This work describes altered glutamine metabolism in CD4+ T cells infected with HIVin vitro.
Liao W, Tan G, Zhu Z, Chen Q, Lou Z, Dong X, et al. Combined metabonomic and quantitative real-time PCR analyses reveal systems metabolic changes in Jurkat T-cells treated with HIV-1 tat protein. J Proteome Res. 2012;11:5109–23. https://doi.org/10.1021/pr300173c.
Bailis W, Shyer JA, Zhao J, Canaveras JCG, Al Khazal FJ, Qu R, et al. Distinct modes of mitochondrial metabolism uncouple T cell differentiation and function. Nature. 2019;571:403–7. https://doi.org/10.1038/s41586-019-1311-3.
• Castellano P, Prevedel L, Valdebenito S, Eugenin EA. HIV infection and latency induce a unique metabolic signature in human macrophages. Sci Rep. 2019;9:3941. https://doi.org/10.1038/s41598-019-39898-5. This study shows that HIV infection of macrophagesin vitroresults in enlarged mitochondria with reduced function without any change in glycolysis.
Barrero CA, Datta PK, Sen S, Deshmane S, Amini S, Khalili K, et al. HIV-1 Vpr modulates macrophage metabolic pathways: a SILAC-based quantitative analysis. PLoS One. 2013;8:e68376. https://doi.org/10.1371/journal.pone.0068376.
Datta PK, Deshmane S, Khalili K, Merali S, Gordon JC, Fecchio C, et al. Glutamate metabolism in HIV-1 infected macrophages: role of HIV-1 Vpr. Cell Cycle. 2016;15:2288–98. https://doi.org/10.1080/15384101.2016.1190054.
Palmer CS, Ostrowski M, Gouillou M, Tsai L, Yu D, Zhou J, et al. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS. 2013;28:297–309. https://doi.org/10.1097/QAD.0000000000000128.
Palmer CS, Anzinger JJ, Zhou J, Gouillou M, Landay A, Jaworowski A, et al. Glucose transporter 1 − expressing proinflammatory monocytes are elevated in combination antiretroviral therapy − treated and untreated HIV + subjects. J Immunol. 2014;193:5595–603. https://doi.org/10.4049/jimmunol.1303092.
Masson JJR, Murphy AJ, Lee MKS, Ostrowski M, Crowe SM, Palmer CS. Assessment of metabolic and mitochondrial dynamics in CD4+ and CD8+ T cells in virologically suppressed HIV-positive individuals on combination antiretroviral therapy. PLoS One. 2017;12:e0183931. https://doi.org/10.1371/journal.pone.0183931.
Masson JJR, Cherry CL, Murphy NM, Sada-Ovalle I, Hussain T, Palchaudhuri R, et al. Polymorphism rs1385129 within Glut1 gene SLC2A1 is linked to poor CD4+ T cell recovery in antiretroviral-treated HIV+ individuals. Front Immunol. 2018;9:900. https://doi.org/10.3389/fimmu.2018.00900.
•• Korencak M, Byrne M, Richter E, Schultz BT, Juszczak P, Ake JA, et al. Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight. 2019;4:e126675. https://doi.org/10.1172/jci.insight.126675. This work utilized Seahorse extracellular flux analysis to assess the metabolism of multiple immune cell types from PLWH and showed that incubation of CD4+ T cells with integrase strand inhibitors decreased oxidative phosphorylation.
Petrovas C, Mueller YM, Dimitriou ID, Altork SR, Banerjee A, Sklar P, et al. Increased mitochondrial mass characterizes the survival defect of HIV-specific CD8+ T cells. Blood. 2007;109:2505–13. https://doi.org/10.1182/blood-2006-05-021626.
Kalinowska M, Bazdar DA, Lederman MM, Funderburg N, Sieg SF. Decreased IL-7 responsiveness is related to oxidative stress in HIV disease. PLoS One. 2013;8:e58764. https://doi.org/10.1371/journal.pone.0058764.
Franchina DG, Grusdat M, Brenner D. B-cell metabolic remodeling and cancer. Trends in cancer. 2018.;4:138–50. https://doi.org/10.1016/j.trecan.2017.12.006.
Mah AY, Cooper MA. Metabolic regulation of natural killer cell IFN-γ production. Crit Rev Immunol. 2016;36:131–47. https://doi.org/10.1615/CritRevImmunol.2016017387.
Batman PA, Miller ARO, Forster SM, Harris JRW, Pinching AJ, Griffin GE. Jejunal enteropathy associated with human immunodeficiency virus infection: quantitative histology. J Clin Pathol. 1989;42:275–81. https://doi.org/10.1136/jcp.42.3.275.
Lim SG, Menzies IS, Lee CA, Johnson MA, Pounder RE. Intestinal permeability and function in patients infected with human immunodeficiency virus: a comparison with coeliac disease. Scand J Gastroenterol. 1993;28:573–80. https://doi.org/10.3109/00365529309096090.
Chung CY, Alden SL, Funderburg NT, Fu P, Levine AD. Progressive proximal-to-distal reduction in expression of the tight junction complex in colonic epithelium of virally-suppressed HIV+ individuals. PLoS Pathog. 2014;10. https://doi.org/10.1371/journal.ppat.1004198.
Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18. https://doi.org/10.1128/CMR.00050-12.
Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. https://doi.org/10.1038/nm1511.
Kristoff J, Haret-Richter G, Ma D, Ribeiro RM, Xu C, Cornell E, et al. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. J Clin Invest. 2014;124:2802–6. https://doi.org/10.1172/JCI75090.
Fitzgerald FC, Lhomme E, Harris K, Kenny J, Doyle R, Kityo C, et al. Microbial translocation does not drive immune activation in ugandan children infected with HIV. J Infect Dis. 2019;219:89–100. https://doi.org/10.1093/infdis/jiy495.
Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, et al. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014;210:1549–54. https://doi.org/10.1093/infdis/jiu305.
Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, et al. Dysbiosis of the gut microbiota is associated with hiv disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193ra91. https://doi.org/10.1126/scitranslmed.3006438.
Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829. https://doi.org/10.1371/journal.ppat.1003829.
Vázquez-Castellanos JF, Serrano-Villar S, Latorre A, Artacho A, Ferrús ML, Madrid N, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015;8:760–72. https://doi.org/10.1038/mi.2014.107.
Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS. 2015;29:2409–18. https://doi.org/10.1097/QAD.0000000000000869.
• Dillon SM, Kibbie J, Lee EJ, Guo K, Santiago ML, Austin GL, et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS. 2017;31:511–21. https://doi.org/10.1097/QAD.0000000000001366. This study shows an inverse relationship between the abundance of butyrate producing bacteria in the colonic mucosae and immune activation in PLWHIn vitroaddition of butyrate decreased immune activation of T cells.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. https://doi.org/10.1038/nature12721.
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–39. https://doi.org/10.1016/j.immuni.2013.12.007.
Säemann MD, Böhmig GA, Österreicher CH, Burtscher H, Parolini O, Diakos C, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14:2380–2. https://doi.org/10.1096/fj.00-0359fje.
Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–52. https://doi.org/10.1073/pnas.1322269111.
Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5:e73. https://doi.org/10.1038/cti.2016.17.
Serrano-Villar S, Vázquez-Castellanos JF, Vallejo A, Latorre A, Sainz T, Ferrando-Martínez S, et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 2017;10:1279–93. https://doi.org/10.1038/mi.2016.122.
Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50:432–445.e7. https://doi.org/10.1016/j.immuni.2018.12.018.
Hoel H, Hove-Skovsgaard M, Hov JR, Gaardbo JC, Holm K, Kummen M, et al. Impact of HIV and type 2 diabetes on gut microbiota diversity, tryptophan catabolism and endothelial dysfunction. Sci Rep. 2018;8:1–9. https://doi.org/10.1038/s41598-018-25168-3.
Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2, 3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. https://doi.org/10.1126/scitranslmed.3000632.
• Qi Q, Hua S, Clish CB, Scott JM, Hanna DB, Wang T, et al. Plasma tryptophan-kynurenine metabolites are altered in human immunodeficiency virus infection and associated with progression of carotid artery atherosclerosis. Clin Infect Dis. 2018;67:235–42. https://doi.org/10.1093/cid/ciy053. This study provides evidence for a linkage between tryptophan catabolism, immune activation and the presence of carotid plaques in PLWH.
Vázquez-Castellanos JF, Serrano-Villar S, Jiménez-Hernández N, Soto Del Rio MD, Gayo S, Rojo D, et al. Interplay between gut microbiota metabolism and inflammation in HIV infection. ISME J. 2018;12:1964–76. https://doi.org/10.1038/s41396-018-0151-8.
Haissman JM, Knudsen A, Hoel H, Kjær A, Kristoffersen US, Berge RK, et al. Microbiota-dependent marker TMAO is elevated in silent ischemia but is not associated with first-time myocardial infarction in HIV infection. J Acquir Immune Defic Syndr. 2016;71:130–6. https://doi.org/10.1097/QAI.0000000000000843.
Haissman JM, Haugaard AK, Ostrowski SR, Berge RK, Hov JR, Trøseid M, et al. Microbiota-dependent metabolite and cardiovascular disease marker trimethylamine-N-oxide (TMAO) is associated with monocyte activation but not platelet function in untreated HIV infection. BMC Infect Dis. 2017;17:1–8. https://doi.org/10.1186/s12879-017-2547-x.
Srinivasa S, Fitch KV, Lo J, Kadar H, Knight R, Wong K, et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS. 2015;29:443–52. https://doi.org/10.1097/QAD.0000000000000565.
Elliott Miller P, Haberlen SA, Brown TT, Margolick JB, DiDonato JA, Hazen SL, et al. Intestinal microbiota-produced trimethylamine-N-oxide and its association with coronary stenosis and HIV serostatus. J Acquir Immune Defic Syndr. 2016;72:114–8. https://doi.org/10.1097/QAI.0000000000000937.
Shan Z, Clish CB, Hua S, Scott JM, Hanna DB, Burk RD, et al. Gut microbial-related choline metabolite trimethylamine-N-oxide is associated with progression of carotid artery atherosclerosis in HIV infection. J Infect Dis. 2018;218:1474–9. https://doi.org/10.1093/infdis/jiy356.
Knudsen A, Christensen TE, Thorsteinsson K, Ghotbi AA, Hasbak P, Lebech AM, et al. Microbiota-dependent marker TMAO is not associated with decreased myocardial perfusion in well-treated HIV-infected patients as assessed by 82 rubidium PET/CT. J Acquir Immune Defic Syndr 2016;72:e83–5. https://doi.org/10.1097/QAI.0000000000001044.
Missailidis C, Neogi U, Stenvinkel P, Trøseid M, Nowak P, Bergman P. The microbial metabolite trimethylamine-N-oxide in association with inflammation and microbial dysregulation in three HIV cohorts at various disease stages. AIDS. 2018;32:1589–98. https://doi.org/10.1097/QAD.0000000000001813.
Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5:e8886. https://doi.org/10.1371/journal.pone.0008886.
Gianella S, Massanella M, Wertheim JO, Smith DM. The sordid affair between human herpesvirus and HIV. J Infect Dis. 2015;212:845–52. https://doi.org/10.1093/infdis/jiv148.
Deayton J, Mocroft A, Wilson P, Emery VC, Johnson MA, Griffiths PD. Loss of cytomegalovirus (CMV) viraemia following highly active antiretroviral therapy in the absence of specific anti-CMV therapy. AIDS. 1999;13:1203–6. https://doi.org/10.1097/00002030-199907090-00008.
Freeman ML, Shive CL, Nguyen TP, Younes SA, Panigrahi S, Lederman MM. Cytokines and T-cell homeostasis in HIV infection. J Infect Dis. 2016;214:S51–7. https://doi.org/10.1093/infdis/jiw287.
Barrett L, Stapleton SN, Fudge NJ, Grant MD. Immune resilience in HIV-infected individuals seronegative for cytomegalovirus. AIDS. 2014;28:2045–9. https://doi.org/10.1097/QAD.0000000000000405.
Lee SA, Sinclair E, Hatano H, Hsue PY, Epling L, Hecht FM, et al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One. 2014;9:e89444. https://doi.org/10.1371/journal.pone.0089444.
Gianella S, Massanella M, Richman DD, Little SJ, Spina CA, Vargas MV, et al. Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4 + T cell activation during antiretroviral treatment. J Virol. 2014;88:7818–27. https://doi.org/10.1128/jvi.00831-14.
•• Christensen-Quick A, Massanella M, Frick A, Rawlings SA, Spina C, Vargas-Meneses M, et al. Subclinical cytomegalovirus DNA is associated with CD4 T cell activation and impaired CD8 T Cell CD107a expression in people living with HIV despite early antiretroviral therapy. J Virol. 2019;93:e00179–19. https://doi.org/10.1128/jvi.00179-19. This work shows that CMV replication, but not EBV replication, is associated with CD4+ T cell activation that persists even after early ART treatment.
Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–83. https://doi.org/10.1093/infdis/jir060.
Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105:3879–84. https://doi.org/10.1073/pnas.0800050105.
Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF. Re-evaluating evolution in the HIV reservoir. Nature. 2017;551:E6–8. https://doi.org/10.1038/nature24634.
Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Kosakovsky Pond SL, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51–6. https://doi.org/10.1038/nature16933.
Ostrowski SR, Katzenstein TL, Pedersen BK, Gerstoft J, Ullum H. Residual viraemia in HIV-1-infected patients with plasma viral load ≤20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol. 2008;68:652–60. https://doi.org/10.1111/j.1365-3083.2008.02184.x.
Mavigenr M, Delobel P, Cazabat M, Dubois M, L’Faqihi-Olive FE, Raymond S, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009;4:e7658. https://doi.org/10.1371/journal.pone.0007658.
• Henrich TJ, Hobbs KS, Hanhauser E, Scully E, Hogan LE, Robles YP, et al. Human immunodeficiency virus type 1 persistence following systemic chemotherapy for malignancy. J Infect Dis. 2017;216:254–62. https://doi.org/10.1093/infdis/jix265. This study demonstrates increased HIV DNA and RNA predominantly in CMV-specific and EBV-specific CD4+ T cells.
Baroncelli S, Galluzzo CM, Pirillo MF, Mancini MG, Weimer LE, Andreotti M, et al. Microbial translocation is associated with residual viral replication in HAART-treated HIV+ subjects with <50copies/ml HIV-1 RNA. J Clin Virol. 2009;46:367–70. https://doi.org/10.1016/j.jcv.2009.09.011.
Maagaard A, Holberg-Petersen M, Løvgården G, Holm M, Olav Pettersen F, Kvale D. Distinct mechanisms for mitochondrial DNA loss in T and B lymphocytes from HIV-infected patients exposed to nucleoside reverse-transcriptase inhibitors and those naive to antiretroviral treatment. J Infect Dis. 2008;198:1474–81. https://doi.org/10.1086/592713.
Wallace ZR, Sanderson S, Simon AK, Dorrell L. Exposure to zidovudine adversely affects mitochondrial turnover in primary T cells. Antivir Res. 2016;133:178–82. https://doi.org/10.1016/j.antiviral.2016.08.002.
Zhao X, Sun K, Lan Z, Song W, Cheng L, Chi W, et al. Tenofovir and adefovir down-regulate mitochondrial chaperone TRAP1 and succinate dehydrogenase subunit B to metabolically reprogram glucose metabolism and induce nephrotoxicity. Sci Rep. 2017;7:1–15. https://doi.org/10.1038/srep46344.
• Yu F, Hao Y, Zhao H, Xiao J, Han N, Zhang Y, et al. Distinct mitochondrial disturbance in CD4+T and CD8+T cells from HIV-infected patients. J Acquir Immune Defic Syndr. 2017;74:206–12. https://doi.org/10.1097/QAI.0000000000001175. This study compares mitochondrial function in CD4+ T cells and CD8+ T cells from people without HIV, ART-naïve and ART-experienced PLWH. The authors show an accumulation of reactive oxygen species in CD8+ T cells in ART-experienced PLWH.
Setzer B, Schlesier M, Walker UA. Effects of didanosine-related depletion of mtDNA in human T lymphocytes. J Infect Dis. 2005;191:848–55. https://doi.org/10.1086/427655.
Selvaraj S, Ghebremichael M, Li M, Foli Y, Langs-Barlow A, Ogbuagu A, et al. Antiretroviral therapy-induced mitochondrial toxicity: potential mechanisms beyond polymerase-γ inhibition. Clin Pharmacol Ther. 2014;96:110–20. https://doi.org/10.1038/clpt.2014.64.
Appay V, Sauce D. Assessing immune aging in HIV-infected patients. Virulence. 2017;8:529–538. https://doi.org/10.1080/21505594.2016.1195536.
Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:e20. https://doi.org/10.1371/journal.pbio.0020020.
Dan JM, Massanella M, Smith DM, Spina CA, Schrier R, Daar ES, et al. Effect of CMV and HIV transcription on CD57 and PD-1 T-cell expression during suppressive ART. J Acquir Immune Defic Syndr. 2016;72:133–7. https://doi.org/10.1097/QAI.0000000000000936,.
Deeks SG. HIV infection, inflammation, Immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. https://doi.org/10.1146/annurev-med-042909-093756.HIV.
Alcaide ML, Parmigiani A, Pallikkuth S, Roach M, Freguja R, Della NM, et al. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One. 2013;8:e63804. https://doi.org/10.1371/journal.pone.0063804.
Desai S, Landay AL. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 2010;7:4–10. https://doi.org/10.1007/s11904-009-0038-4.Early.
Miller CJ, Baker JV, Bormann AM, Erlandson KM, Hullsiek KH, Justice AC, et al. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One. 2014;9:e95061. https://doi.org/10.1371/journal.pone.0095061.
Pinto DSM, da Silva MJLV. Cardiovascular disease in the setting of human immunodeficiency virus infection. Curr Cardiol Rev. 2018;14:25–41. https://doi.org/10.2174/1573403x13666171129170046.
Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS ONE. 2017;12:e0176686. https://doi.org/10.1371/journal.pone.0176686.
Cerrato E, D’Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–6. https://doi.org/10.1093/eurheartj/ehs471.
Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–22. https://doi.org/10.1161/CIRCULATIONAHA.113.001719.
Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, et al. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis. 2015;212:1544–51. https://doi.org/10.1093/infdis/jiv274.
Hsu JC, Li Y, Marcus GM, Hsue PY, Scherzer R, Grunfeld C, et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol. 2013;61:2288–95. https://doi.org/10.1016/j.jacc.2013.03.022.
Janda S, Quon BS, Swiston J. HIV and pulmonary arterial hypertension: a systematic review. HIV Med. 2010;11:620–34. https://doi.org/10.1111/j.1468-1293.2010.00829.x.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. https://doi.org/10.1038/nature10146.
Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. https://doi.org/10.1371/journal.pmed.0050203.
• Siedner MJ, Kim JH, Nakku RS, Bibangambah P, Hemphill L, Triant VA, et al. Persistent immune activation and carotid atherosclerosis in HIV-infected Ugandans receiving antiretroviral therapy. J Infect Dis. 2016;213:370–8. https://doi.org/10.1093/infdis/jiv450. This study highlights that the association of immune activation and CVD in PLWH also applies to the low- and middle-income country setting.
Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;60:359–68. https://doi.org/10.1097/QAI.0b013e31825b03be.Potential.
Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–63. https://doi.org/10.1093/infdis/jiq071.
Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–36. https://doi.org/10.1093/infdis/jir520.
Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–7. https://doi.org/10.1182/blood-2009-03-210179.
•• Schechter ME, Andrade BB, He T, Richter GH, Tosh KW, Policicchio BB, et al. Inflammatory monocytes expressing tissue factor drive SIV and HIV coagulopathy. Sci Transl Med. 2017;9:eaam5441. https://doi.org/10.1126/scitranslmed.aam5441. This study demonstrates a linkage between, microbial translocation, innate immune activation and coagulopathy in an SIV model.
Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–83. https://doi.org/10.1097/QAD.0b013e3280108704.
Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012;205:1788–96. https://doi.org/10.1093/infdis/jis276.
Knudsen A, Kristoffersen US, Panum I, Hansen YB, Skottrup PD, Hasbak P, et al. Coronary artery calcium and intima-media thickness are associated with level of cytomegalovirus immunoglobulin G in HIV-infected patients. HIV Med. 2019;20:60–2. https://doi.org/10.1111/hiv.12672.
•• Anzinger JJ, Butterfield TR, Gouillou M, McCune JM, Crowe SM, Palmer CS. Glut1 expression level on inflammatory monocytes is associated with markers of cardiovascular disease risk in HIV-infected individuals. J Acquir immune Defic Syndr. 2018;77:e28–e30. https://doi.org/10.1097/QAI.0000000000001559. This study provides a link between immunometabolism and cardiovascular disease in treated PLWH.
•• Butterfield TR, Hanna DB, Kaplan RC, Kizer JR, Durkin HG, Young MA, et al. Increased glucose transporter-1 expression on intermediate monocytes from HIV-infected women with subclinical cardiovascular disease. AIDS. 2017;31:199–205. https://doi.org/10.1097/QAD.0000000000001320. This study demonstrates a specific association of inflammatory monocyte immunometabolism and subclinical cardiovascular disease in treated women with HIV.
•• Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res Care. 2017;5:e000304. https://doi.org/10.1136/bmjdrc-2016-000304. This study describes the increased prevalence of diabetes mellitus in a large cohort of PLWH compared to uninfected controls. The authors show that this increased prevalence in PLWH is present even without traditional risk factors of obesity and increased age.
Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. https://doi.org/10.1097/01.QAI.0000179459.31562.16.
Prioreschi A, Munthali RJ, Soepnel L, Goldstein JA, Micklesfield LK, Aronoff DM, et al. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta-analysis. BMJ Open. 2017;7:e013953. https://doi.org/10.1136/bmjopen-2016-013953.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. https://doi.org/10.1038/nri2925.
Shikuma CM, Chow DC, Gangcuangco LMA, Zhang G, Keating SM, Norris PJ, et al. Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS One. 2014;9:e90330. https://doi.org/10.1371/journal.pone.0090330.
Butterfield TR, Hanna DB, Kaplan RC, Kizer JR, Durkin HG, Young MA, et al. HIV+ women with diabetes mellitus exhibit CD4+ T lymphocyte immunometabolic dysfunction. In: International AIDS Society Conference on HIV Science. Mexico City, Mexico; 2019.
• Couturier J, Agarwal N, Nehete PN, Baze WB, Barry MA, Jagannadha Sastry K, et al. Infectious SIV resides in adipose tissue and induces metabolic defects in chronically infected rhesus macaques. Retrovirology. 2016;13:30. https://doi.org/10.1186/s12977-016-0260-2. This SIV study describes the association of GLUT4 expression on adipocytes with increased percentage of peripheral CD8+ T cells and increased inflammatory cytokines. This provides a potential link between diabetes mellitus and inflammation.
Dye CK, Corley MJ, Li D, Khadka VS, Mitchell BI, Sultana R, et al. Comparative DNA methylomic analyses reveal potential origins of novel epigenetic biomarkers of insulin resistance in monocytes from virally suppressed HIV-infected adults. Clin Epigenetics. 2019;11:1–19. https://doi.org/10.1186/s13148-019-0694-1.
Wang Q, Wu H. T cells in adipose tissue: critical players in Immunometabolism. Front Immunol. 2018;9:2509. https://doi.org/10.3389/fimmu.2018.02509.
Shikuma CM, Gangcuango LMA, Killebrew DA, LiButti DE, Chow DC, Nakamoto BK, et al. The role of HIV and monocytes/macrophages in adipose tissue biology. J Acquir Immune Defic Syndr. 2015;65:151–9. https://doi.org/10.1097/01.qai.0000435599.27727.6c.The.
Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X, et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS. 2015;29:667–74. https://doi.org/10.1097/QAD.0000000000000599.
• Wanjalla CN, WJ MD, Barnett L, Simmons JD, Furch BD, Lima MC, et al. Adipose tissue in persons with HIV is enriched for CD4+ T effector memory and T effector memory RA+ cells, which show higher CD69 expression and CD57, CX3CR1, GPR56 co-expression with increasing glucose intolerance. Front Immunol. 2019;10:408. https://doi.org/10.3389/fimmu.2019.00408. This study of PLWH demonstrates increasing proportions of activated tissue resident CD4+ T cells in adipose tissue with increasing glucose intolerance, providing a link between immune activation and diabetes mellitus.
Moon JY, Zolnik CP, Wang Z, Qiu Y, Usyk M, Wang T, et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine. 2018;37:392–400. https://doi.org/10.1016/j.ebiom.2018.10.037.
Bailin SS, Jenkins CA, Petucci C, Culver JA, Shepherd BE, Fessel JP, et al. Lower concentrations of circulating medium and long-chain acylcarnitines characterize insulin resistance in persons with HIV. AIDS Res Hum Retrovir. 2018;34:536–43. https://doi.org/10.1089/aid.2017.0314.
Harrison ML, Wolfe AS, Fordyce J, Rock J, García AA, Zuñiga JA. The additive effect of type 2 diabetes on fibrinogen, von Willebrand factor, tryptophan and threonine in people living with HIV. Amino Acids. 2019;51:783–93. https://doi.org/10.1007/s00726-019-02715-4.
Brunt SJ, Cysique LA, Lee S, Burrows S, Brew BJ, Price P. Short communication: do cytomegalovirus antibody levels associate with age-related syndromes in HIV patients stable on antiretroviral therapy? AIDS Res Hum Retrovir. 2016;32:567–72. https://doi.org/10.1089/aid.2015.0328.
Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33:2376–83. https://doi.org/10.1200/JCO.2014.59.5967.
Coghill AE, Han X, Suneja G, Lin CC, Jemal A, Shiels MS. Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer. 2019;125:2868–76. https://doi.org/10.1002/cncr.32158.
Engels EA, Yanik EL, Wheeler W, Gill MJ, Shiels MS, Dubrow R, et al. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis. 2017;65:636–43. https://doi.org/10.1093/cid/cix392.
Mitsuyasu RT. Non-AIDS-defining cancers. Top Antivir Med. 2014;22:660–5. https://doi.org/10.1097/QCO.0b013e3283213080.
Kirk GD, Merlo C, O’Driscoll P, Mehta SH, Galai N, Vlahov D, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–10. https://doi.org/10.1086/518606.
Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383–8. https://doi.org/10.1200/JCO.2005.03.4413.
Borges ÁH, Silverberg MJ, Wentworth D, Grulich AE, Fätkenheuer G, Mitsuyasu R, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS. 2013;27:1433–41. https://doi.org/10.1097/QAD.0b013e32835f6b0c.
Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23:875–85. https://doi.org/10.1097/QAD.0b013e328329216a.
Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV. 2017;4:e67–73. https://doi.org/10.1016/S2352-3018(16)30215-6.
Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z, et al. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front Immunol. 2018;9:151. https://doi.org/10.3389/fimmu.2018.00151.
Comito G, Iscaro A, Bacci M, Morandi A, Ippolito L, Parri M, et al. Lactate modulates CD4 + T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. 2019;38:3681–95. https://doi.org/10.1038/s41388-019-0688-7.
Raychaudhuri D, Bhattacharya R, Sinha BP, Liu CSC, Ghosh AR, Rahaman O, et al. Lactate induces pro-tumor reprogramming in intratumoral plasmacytoid dendritic cells. Front Immunol. 2019;10:1878. https://doi.org/10.3389/fimmu.2019.01878.
Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–86. https://doi.org/10.1093/gerona/62.11.1279.
Erlandson KM, Ng DK, Jacobson LP, Margolick JB, Dobs AS, Palella FJ, et al. Inflammation, immune activation, immunosenescence, and hormonal biomarkers in the frailty-related phenotype of men with or at risk for HIV infection. J Infect Dis. 2017;215:228–37. https://doi.org/10.1093/infdis/jiw523.
Margolick JB, Bream JH, Martínez-Maza O, Lopez J, Li X, Phair JP, et al. Frailty and circulating markers of inflammation in HIV+ and HIV2 men in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2017;74:407–17. https://doi.org/10.1097/QAI.0000000000001261.
• Yeoh HL, Cheng AC, Cherry CL, Weir JM, Meikle PJ, Hoy JF, et al. Immunometabolic and lipidomic markers associated with the frailty index and quality of life in aging HIV+ men on antiretroviral therapy. EBioMedicine. 2017;22:112–21. https://doi.org/10.1016/j.ebiom.2017.07.015. This study shows that frail men living with HIV have increased expression of GLUT1 on monocytes and increased markers of innate immune activation.
Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–96. https://doi.org/10.1212/WNL.0b013e318200d727.
McGuire JL, Gill AJ, Douglas SD, Kolson DL. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neuro-Oncol. 2015;21:439–48. https://doi.org/10.1007/s13365-015-0333-3.
Ancuta P, Kamat A, Kunstman KJ, Kim E-Y, Autissier P, Wurcel A, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. https://doi.org/10.1371/journal.pone.0002516.
Letendre S, Bharti A, Perez-Valero I, Hanson B, Franklin D, Woods SP, et al. Higher anti-cytomegalovirus immunoglobulin G concentrations are associated with worse neurocognitive performance during suppressive antiretroviral therapy. Clin Infect Dis. 2018;67:770–7. https://doi.org/10.1093/cid/ciy170.
Zamudio-Rodríguez A, Belaunzarán-Zamudio PF, Sierra-Madero JG, Cuellar-Rodríguez J, Crabtree-Ramírez BE, Alcala-Zermeno JL, et al. Association between frailty and HIV-associated neurodegenerative disorders among older adults living with HIV. AIDS Res Hum Retrovir. 2018;34:449–55. https://doi.org/10.1089/aid.2017.0100.
Sacktor N. Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol. 2018;24:141–5. https://doi.org/10.1007/s13365-017-0556-6.
Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr. 2012;61:600–5. https://doi.org/10.1097/QAI.0b013e31827303d5.
Duffau P, Ozanne A, Bonnet F, Lazaro E, Cazanave C, Blanco P, et al. Multimorbidity, age-related comorbidities and mortality: association of activation, senescence and inflammation markers in HIV adults. AIDS. 2018;32:1651–60. https://doi.org/10.1097/QAD.0000000000001875.
Funding
This work was supported in part by a UM1 AI106701 award (Alan Landay) and the Office of the Principal of the University of the West Indies, Mona (Joshua Anzinger).
As a Global Infectious Diseases Scholar, Tiffany Butterfield received mentored research training in the development of this manuscript. This training was supported in part by the University at Buffalo Clinical and Translational Science Institute award UL1TR001412 and the Global Infectious Diseases Research Training Program award D43TW010919. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Clinical and Translational Science Institute or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human subjects performed by the authors have been previously published and complied with all applicable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on HIV Pathogenesis and Treatment
Rights and permissions
About this article
Cite this article
Butterfield, T.R., Landay, A.L. & Anzinger, J.J. Dysfunctional Immunometabolism in HIV Infection: Contributing Factors and Implications for Age-Related Comorbid Diseases. Curr HIV/AIDS Rep 17, 125–137 (2020). https://doi.org/10.1007/s11904-020-00484-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-020-00484-4