Abstract
Interpretation of microbicide trial results has been compromised by challenges to accurate measurement of product adherence and sexual risk behaviors. This article provides an evaluation of the methods used to measure adherence and other sensitive behaviors relevant to assessment of product safety and effectiveness in recently completed trials of vaginal and rectal microbicides. We review the strengths and limitations of existing and novel behavioral measurement strategies and provide recommendations for future trial design with the goal of facilitating the development and identification of safe and effective microbicides for HIV prevention.

Similar content being viewed by others
Notes
Microbicide Trials Network statements on decision to discontinue use of oral Tenofovir tablets and Tenofovir gel in VOICE, a major HIV prevention study in women. http://www.mtnstopshiv.org/node/3909: Microbicide Trials Network; 2011. Note that the oral Truvada and placebo arms continued and were completed on schedule in August 2012 (http://www.mtnstopshiv.org/studies/70).
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Verma NA, Lee AC, Herold BC, et al. Topical prophylaxis for HIV prevention in women: becoming a reality. Curr HIV/AIDS Rep. 2011;8:104–13.
•• Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. This paper presents the results of CAPRISA 004, the only trial demonstrating effectiveness of a microbicide gel to date.
van der Straten A, Van Damme L, Haberer JE, et al. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–9.
Weiss HA, Wasserheit JN, Barnabas RV, et al. Persisting with prevention: the importance of adherence for HIV prevention. Emerg Themes Epidemiol. 2008;5:1–7.
Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410.
Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99.
• Karim SSA, Kashuba ADM, Werner L, et al. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–81. This study showed the relationship between drug level in vaginal fluids and protection from HIV acquisition in the CAPRISA 004 trial.
Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22.
Institute of Medicine. Design considerations: adherence. In: Lagakos SW, Gable A, editors. Methodological challenges in biomedical HIV prevention trials. Washington, DC: Natl Academy Press; 2008. p. 119–47.
• Amico K. Adherence to preexposure chemoprophylaxis: the behavioral bridge from efficacy to effectiveness. Curr Opin HIV AIDS. 2012;7:542–48. This review focuses on recent Oral PrEP trials and the behavioral aspects of PrEP adherence. It proposes a behavioral agenda for both oral PrEP research and practice.
McGowan I, Taylor DJ. Heterosexual anal intercourse has the potential to cause a significant loss of power in vaginal microbicide effectiveness studies. Sex Transm Dis. 2010;37:361–4.
Masse BR, Boily MC, Dimitrov D, et al. Efficacy dilution in randomized placebo-controlled vaginal microbicide trials. Emerg Themes Epidemiol. 2009;6(5).
• Muchomba FM, Gearing RE, Simoni JM, et al. State of science of adherence in pre-exposure prophylaxis and microbicide trials. J Acquir Immune Defic Syndr. 2012; doi:10.1097/QAI.0b013e31826f9962. This is a review of adherence in all published oral PrEP and microbicide trials to date.
Greene E, Batona G, Hallad J, et al. Acceptability and adherence of a candidate microbicide gel among high-risk women in Africa and India. Cult Health Sex. 2010;12:739–54.
Van Damme L, Govinden R, Mirembe FM, et al. Lack of Effectiveness of Cellulose Sulfate Gel for the Prevention of Vaginal HIV Transmission. N Engl J Med. 2008;359:463–72.
McCormack S, Ramjee G, Kamali A, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376:1329–37.
Abdool Karim SS, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5 % PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25:957–66.
• Pool R, Montgomery CM, Morar NS, et al. A mixed methods and triangulation model for increasing the accuracy of adherence and sexual behaviour data: the Microbicides Development Programme. PloS One. 2010;5:e11600. This paper contributes to the triangulation methodology of various adherence measures.
Pool R, Montgomery CM, Morar NS, et al. Assessing the accuracy of adherence and sexual behaviour data in the MDP301 vaginal microbicides trial using a mixed methods and triangulation model. PLoS One. 2010;5:e11632.
Tolley EE, Tsui S, Mehendale S, et al. Predicting product adherence in a topical microbicide safety trial in Pune, India. AIDS Behav. 2011;16:1808–15.
Hillier S. Safety and acceptability of daily and coitally dependent use of 1 % tenofovir over 6 months of use. Proceedings of the International Microbicides Conference; New Dehli, India, Feb 24–27, 2008.
Anton PA, Cranston RD, Kashuba A, et al. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic and pharmacodynamic study of tenofovir 1 % gel compared to oral tenofovir disoproxil fumerate. AIDS Res Hum Retroviruses. 2012 (not available - ahead of print).
McGowan I, Hoesley C, Andrew P, et al. MTN-007: a phase 1 randomized, double-blind, placebo-controlled, rectal safety and acceptability study of tenofovir 1 % gel. Proceedings of the Conference on Retroviruses and Opportunistic Infections; Seattle, March 5–8, 2012.
Anton PA, Saunders T, Elliott J, et al. First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 Gel with a novel index of ex vivo efficacy. PLoS One. 2011;6:e23243.
• Cohen CR, Brown J, Moscicki A-B, et al. A phase I randomized placebo controlled trial of the safety of 3 % SPL7013 Gel (VivaGel) in healthy young women administered twice daily for 14 days. PLoS One. 2011;6:e16258. This phase I microbicide trial used multiple measures of adherence, including a dye stain assay (DSA). This is the only microbicide trial reported in the past 2 years to use a DSA. They also used biomarkers to assess sexual abstinence during the trial.
McGowan I, Gomez K, Bruder K, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). AIDS. 2011;25:1057–64.
• Minnis AM, Gandham S, Richardson BA, et al. Adherence and acceptability in MTN 001: a randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women. AIDS Behav. 2012. doi:10.1007/s10461-012-0333-8. This phase II trial is the first to directly compare 2 formulations (oral and vaginal) of the same product (tenofovir); and among the first to use drug levels as a primary adherence outcome for oral dosing. They also conducted a direct comparison between biomarker-based and self-reported adherence.
Hendrix C, Minnis A, Guddera V, et al. MTN-001: a phase 2 cross-over study of daily oral and vaginal TFV in healthy, sexually active women results in significantly different product acceptability and vaginal tissue drug concentrations. Conference on Retroviruses and Opportunistic Infections; February 28; Boston, MA. 2011.
Isaacs M, Albertse M, Hellström E, et al. Daily monitored adherence (DMA) in a microbicide safety trial - IPM 014B. Proceedings of the International Microbicides Conference; Sydney, Australia, April 15–18, 2012.
Montgomery E, van der Straten A, Woodsong C, et al. Daily use of two dapivirine vaginal gels in the United States: adherence and acceptability. Proceedings of the International Microbicides Conference; Sydney, Australia, April 15–18, 2012.
Nel AM, Coplan P, Smythe SC, et al. Pharmacokinetic assessment of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS Res Hum Retrovir. 2010;26:1181–90.
Nel AM, Smythe SC, Habibi S, et al. Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs Hydroxyethyl cellulose-based universal placebo gel. J Acquir Immune Defic Syndr. 2010;55:161–9.
Adudans MK, Gitome SW, Njoroge BN, et al. Daily monitored adherence (DMA): optimizing adherence in a vaginal microbicide study in Kisumu, Kenya. Proceedings of the International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention; Rome, Italy, July 17–20, 2011.
Phase III trial of dapivirine ring begins in Africa: ASPIRE testing new HIV prevention approach for women. Available at: http://www.mtnstopshiv.org/node/4546: Accessed: Microbicides Trial Network, 2012.
First efficacy trial of a microbicide ring to prevent HIV is underway: the ring study to assess IPM’s monthly ARV ring for women. Available at: http://www.ipmglobal.org/publications/first-efficacy-trial-microbicide-ring-prevent-hiv-underway: Accessed: International Partnership for Microbicides, Inc. 2012.
van der Straten A, Woodsong C, Montgomery E, et al. High adherence and acceptability of a monthly Dapivirine vaginal ring for HIV prevention in Africa. Proceedings of the International Microbicides Conference; Sydney, Australia, April 15–18, 2012.
Nel A, Kamupira M, Woodsong C, et al. Safety, acceptability and pharmacokinetic assessment (adherence) of monthly Dapivirine Vaginal Microbicide Rings (Ring-004) for HIV prevention. Proceedings of the Conference on Retroviruses and Opportunistic Infections; Seattle, WA, March 5–8, 2012.
Nel A, Habibi S, Smythe S, et al. Pharmacokinetic and safety assessment of monthly anti-HIV dapivirine vaginal microbicide rings with multiple dosing. Proceedings of the Conference on Retroviruses and Opportunistic Infections; Boston, MA, Feb 27–March 2, 2011.
Kiser PF, Johnson TJ, Clark JT. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 2012;14:62–77.
Montgomery ET, van der Straten A, Cheng H, et al. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS Behav. 2012;7:1787–98.
Montgomery ET, Woodsong C, Musara P, et al. An acceptability and safety study of the Duet cervical barrier and gel delivery system in Zimbabwe. J Int AIDS Soc. 2010;13:30.
Woodsong C, Alleman P. Sexual pleasure, gender power and microbicide acceptability in Zimbabwe and Malawi. AIDS Educ Prev. 2008;20:171–87. Epub 2008/04/25.
Woodsong C, Alleman P, Musara P, et al. Preventive misconception as a motivation for participation and adherence in microbicide trials: evidence from female participants and male partners in Malawi and Zimbabwe. AIDS Behav. 2012;16:785–90.
Nel AM, Mitchnick LB, Risha P, et al. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J Womens Health (Larchmt). 2011;20:1207–14.
Vrijens B, Urquhart J. Patient adherence to prescribed antimicrobial drug dosing regimens. J Antimicrob Chemother. 2005;55:616–27.
•• Blaschke TF, Osterberg L, Vrijens B, et al. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. This thorough review of the treatment adherence literature presents a new framework for adherence assessment and standardized definitions for the components of adherence.
Williams A, Amico K, Bova C, et al. A proposal for quality standards for measuring medication adherence in research. AIDS Behav. 2012. doi:10.1007/s10461-012-0172-7.
Meyer J, Summers B, Lentsoane P, et al. Is a simple self-rating or visual analogue scale more accurate than prescription refill data, as an indicator of non-adherence in a resource-limited setting in South Africa. Proceedings of the International Conference on HIV Treatment and Prevention Adherence; Miami, FL, June 3–5, 2012.
Lu M, Safren S, Skolnik P, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12:86–94.
Minnis AM, Steiner MJ, Gallo MF, et al. Biomarker validation of reports of recent sexual activity: results of a randomized controlled study in Zimbabwe. Am J Epidemiol. 2009;170:918–24.
Ghanem KG, Melendez JH, McNeil-Solis C, et al. Condom use and vaginal Y-chromosome detection: the specificity of a potential biomarker. Sex Transm Dis. 2007;34:620–3.
Carballo-Dieguez A, Giguere R, Dolezal C, et al. "Tell Juliana": acceptability of the candidate microbicide VivaGel((R)) and two placebo gels among ethnically diverse, sexually active young women participating in a phase 1 microbicide study. AIDS Behav. 2011;7:1761–74.
Katzen L, Abbott S, Friedland B, et al. An evaluation of two methods for daily reporting of adherence in a placebo gel trial in southern India. Proceedings of the International Microbicides Conference; Sydney, Australia, April 15–18, 2012.
Curran K, Mugo N, Kurth A, et al. A pilot study of daily SMS surveys of sexual behavior and PrEP use among Kenyan HIV discordent couples. Proceedings of the International Conference on HIV Treatment and Prevention Adherence; Miami, FL, June 4, 2012.
Mutua G, Sanders E, Mugo P, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7:e33103.
Horvath T, Azman H, Kennedy GE, et al. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012;3:1–37.
Haberer J, Baeten J, Celum C, et al. Near perfect early adherence to antiretroviral PrEP againstHIV infection among HIV serodiscordant couples as determined by multiple measures: preliminary data from the partners PrEP study. Proceedings of the Conference on Retroviruses and Opportunistic Infections; Boston, MA, Feb 27–Mar 2, 2011.
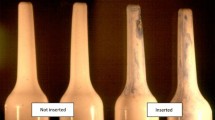
• Moench TR, O'Hanlon DE, Cone RA. Evaluation of microbicide gel adherence monitoring methods. Sex Transm Dis. 2012;39:335–40. This methodological study compared different applicator insertion tests. They developed a simple UV light based applicator test, which they combined with electronic monitoring (MEMS) to monitor quality of execution and applicator insertion.
van der Straten A, Montgomery E, Pillay D, et al. Feasibility, performance, and acceptability of the Wisebag for potential monitoring of daily gel applicator use in Durban, South Africa. AIDS Behav. (in press).
Gengiah T, Mansoor L, Naidoo A, et al. The “Wisebag”: an innovative strategy for enhancing measurement of microbicide gel use in clinical trials. Proceedings of the International Microbicides Conference; Pittsburgh, PA, May 22–25, 2010.
Vitality. Introducing GlowCaps. Available at: http://www.vitality.net/glowcaps_howglowcapswork.html; available at: http://www.vitality.net/glowcaps_howglowcapswork.html. Accessed October 4, 2012.
Moulding T, Ellis D. Electronic medication monitors: status of development and potential for improving effective TB treatment programs Available at: http://www.medicationmonitors.net/. Accessed 2012.
Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–87.
Katzen LL, Fernandez-Romero JA, Sarna A, et al. Validation of a dye stain assay for vaginally inserted hydroxyethylcellulose-filled microbicide applicators. Sex Transm Dis. 2011;38:1050–5.
Mauck CK, Schwartz JL. Dyeing to know: the use of vaginal applicator staining and other techniques to assess adherence to product use in microbicide trials. Sex Transm Dis. 2012;39:713–5.
Morey TE, Wasdo S, Wishin J, et al. Feasibility of a breath test for monitoring adherence to vaginal administration of antiretroviral microbicide gels. J Clin Pharmacol. 2012. doi:10.1177/0091270011434157.
van der Straten A, Wasdo S, Rivett K, et al. A novel breath-based technology to assess concurrent use of vaginal gel and condom. Proceedings of the International AIDS Society Conference; Rome, Italy, July 17–20, 2011.
Au-Yeung KY, Moon GD, Robertson TL, et al. Early clinical experience with networked system for promoting patient self-management. Am J Manag Care. 2011;17:e277–87.
FDA approves digestible microchips to be placed in pills. Available at: http://www.medscape.com/viewarticle/768665?src=nldne%3Fsrc%3Dstfb: Accessed: Medscape Medical News; 2012.
•• Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Sci Transl Med. 2012;4(151):151ra25. Epub 2012/09/14. This is the first published study to establish a quantitative relationship between active intracellular drug concentration in peripheral blood mononuclear cells and level of protection from HIV-1 acquisition, using data from the oral PrEP trial iPrEx and from a separate study, the STRAND trial, which used directly observed oral dosing.
Liu A. What we might learn about PrEP adherence from biomarkers: promise and limitations. Proceedings of the International Microbicide Conference; Sydney, Australia, April 15–18, 2012.
Schwartz JL, Rountree W, Kashuba ADM, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1 % tenofovir gel. PLoS One. 2011;6:e25974.
Romano J, Variano B, Coplan P, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retrovir. 2009;25:483–8.
Singer R, Mawson P, Derby N, et al. An intravaginal ring that releases the NNRTI MIV-150 reduces SHIV transmission in macaques. Sci Transl Med. 2012;4:150ra23–ra23.
Keller MJ, Madan RP, Torres NM, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1 % tenofovir gel. PLoS One. 2011;6:e16475.
de Bruin M, Viechtbauer W. The meaning of adherence when behavioral risk patterns vary: obscured use and method-effectiveness in HIV-prevention trials. PLoS One. 2012;7:e44029.
Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med. 2011;8:e1000416.
Martin Hilber A, Chersich M, van de Wijgert J, et al. Vaginal practices, microbicides and HIV:what do we need to know? Sex Transm Dis. 2007;83:505–8.
Martin Hilber A, Kenter E, Redmond S, et al. Vaginal practices as women's agency in Sub-Saharan Africa: A synthesis of meaning and motivation through meta-ethnography. Soc Sci Med. 2012;74:1311–23.
Gorbach PM, Mensch BS, Husnik M, et al. Effect of computer-assisted interviewing on self-reported sexual behavior data in a microbicide clinical trial. AIDS Behav. 2012. doi:10.1007/s10461-012-0302-2.
• Mensch BS, Hewett PC, Abbott S, et al. Assessing the reporting of adherence and sexual activity in a simulated microbicide trial in South Africa: an interview mode experiment using a placebo gel. AIDS Behav. 2011;15:407–21. This methodological experiment compared ACASI to FTFI modes for self-reported behaviors, including gel use, and biological markers of product adherence and sexual exposure.
Mauck CK. Biomarkers for evaluating vaginal microbicides and contraceptives: discovery and early validation. Sex Transm Dis. 2009;36:S73–5.
Walsh T, Warner L, Macaluso M, et al. Prostate-specific antigen as a biomarker of condom failure: comparison of three laboratory assays and self-reported condom use problems in a randomized trial of female condom performance. Contraception. 2012;86:55–61.
Old J, Schweers BA, Boonlayangoor PW, et al. Developmental validation of RSID™ semen: a lateral flow immunochromatographic strip test for the forensic detection of human semen*. J Forensic Sci. 2012;57:489–99.
Ware NC, Wyatt MA, Haberer JE, et al. What's love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59:463.
Nunn A, McCormack S, Crook AM, et al. Microbicides Development Programme: design of a phase III trial to measure the efficacy of the vaginal microbicide PRO 2000/5 for HIV prevention. Trials. 2009;10:99.
Stirratt MJ, Gordon CM. Adherence to biomedical HIV prevention methods: considerations drawn from HIV treatment adherence research. Curr HIV/AIDS Rep. 2008;5:186–92.
Auerbach JD, Coates TJ. HIV prevention research: accomplishments and challenges for the third decade of AIDS. Am J Public Health. 2000;90:1029.
Friedland B, Abbott S, Sarna A, et al. Does advance knowledge of biomarkers improve adherence or the reporting of sexual activity, gel and condom use among clinical trial participants? Results from a placebo gel trial in Andhra Pradesh, India. Proceedings of the HIV/AIDS Network Coordination (HANC) Behavioral Science Working Group Meeting; Miami, FL, June 6, 2012.
Thomsen SC, Gallo MF, Ombidi W, et al. Randomised controlled trial on whether advance knowledge of prostate-specific antigen testing improves participant reporting of unprotected sex. Sex Transm Infect. 2007;83:419–20.
Castillo-Mancilla J, Zheng J-H, Bushman L, et al. Pharmacology of tenofovir, tenofovir-DP, and emtricitabine in RBC and DBS. Proceedings of the Conference on Retroviruses and Opportunistic Infections; Seattle, WA, March 5–8, 2012.
DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200.
Nel A, Kamupira M, Hetro C, et al. Safety and pharmacokinetics trial of Dapivirine Matrix Vaginal Ring. International AIDS Conference; Vienna, Austria, July 18–23, 2010.
Joglekar NS, Joshi SN, Deshpande SS, et al. Acceptability and adherence: findings from a Phase II study of a candidate vaginal microbicide, 'Praneem polyherbal tablet', in Pune, India. Trans R Soc Trop Med Hyg. 2010;104:412–5.
Joshi SN, Dutta S, Kumar BK, et al. Expanded safety study of Praneem polyherbal vaginal tablet among HIV-uninfected women in Pune, India: a phase II clinical trial report. Sex Transm Infect. 2008;84:343–7.
Lees S, Cook C, Vallely A, et al. Comparison of sexual behavior data collected using a coital diary and a clinic-based interview during a microbicide pilot study in Mwanza, Tanzania. Sex Transm Dis. 2010;37:497–501.
Hemmerling A, Harrison WG, Brown JM, et al. Trypan blue staining to determine vaginal exposure in two types of plastic vaginal applicators containing two different microbicide formulations. Sex Transm Dis. 2012;39:710–2.
Naditz A. Medication compliance—helping patients through technology: modern “smart” pillboxes keep memory-short patients on their medical regimen. Telemed J E Health. 2008;14:875–80.
Acknowledgments
The authors would like to thank the International Partnership for Microbicides (IPM) and Drs. Sue Napierala Mavedzenge and Christine Mauck for their review of an earlier version of the manuscript. Support for preparation of this manuscript was provided in part by a Professional Development Award from RTI International, for Ariane van der Straten. Alexandra Minnis’s contributions to the manuscript were supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development at the National Institutes of Health (K01 HD047434). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of RTI or of the NIH. Drs. van der Straten, Minnis and Montgomery are members of the Behavioral Research Working Group of the Microbicide Trial Network. Dr. van der Straten is Co-Investigator on the VOICE and ASPIRE trials and Dr. Minnis on the MTN-001 trial. Dr. van der Straten conceived the idea and led the writing of this manuscript. All authors wrote at least 1 section of the manuscript and contributed to editing and modifying the content of the manuscript as a whole.
Disclosure
A. van der Straten: none; E. T. Montgomery: none; M. Hartmann: none; A. Minnus: conference travel and accommodations from Microbicides Trial Network.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Straten, A., Montgomery, E.T., Hartmann, M. et al. Methodological Lessons from Clinical Trials and the Future of Microbicide Research. Curr HIV/AIDS Rep 10, 89–102 (2013). https://doi.org/10.1007/s11904-012-0141-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-012-0141-9