Abstract
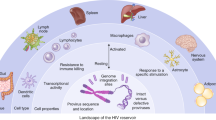
Despite the tremendous advances in antiretroviral combination therapy over the last decade, eradication of HIV from the infected organism is still an elusive goal. Lifelong therapy is associated with potential long-term toxicity, adherence problems, and development of drug resistance. Thus, gene therapy approaches targeting viral eradication are still attractive. Here a number of studies have failed to show a clear clinical benefit yet. Current approaches were mainly limited by a low number of transduced cells and genotoxicity. The use of new vector systems and the right choice of target cells and improved transduction protocols may overcome these obstacles. Recent reports on the use of newly developed transgenes either allowing for an enrichment of transduced cells by an in vivo selection advantage or restoration of a functional immune system which is resistant to HIV infection nourished the hope for continuous progress in this field. Indeed the intriguing finding that HIV seems to be eradicated in an individual case study after stem cell transplantation with a mutant coreceptor (CCR5 delta 32 deletion) underlines the proof of the concept.
Similar content being viewed by others
References
Papers of particular interests, published recently, have been highlighted as:• Of importance •• Of major importance
Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105:3879–84.
Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–714.
van Lunzen J, Hoffmann C. Virological rebound and its consequences during treatment interruption. Curr Opin HIV & AIDS. 2007;(2):1–5 5.
Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300.
Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7.
van Lunzen J. How will CCR5 antagonists influence the recommendations for the antiretroviral treatment of HIV infection. Eur J Med Res. 2007;12:1–6.
Murray JM, Emery S, Kelleher AD, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21:2315–21.
Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 16:460–5.
Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 7.
Delaugerre C, Charreau I, Braun J, et al. Time course of total HIV-1 DNA and 2-long-terminal repeat circles in patients with controlled plasma viremia switching to a raltegravir-containing regimen. Aids. 24:2391–5.
McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 50:912–9.
Yukl SA, Shergill AK, McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. Aids.
Walker R, Blaese RM, Carter CS, Chang L, Klein H, Lane HC, et al. A study of the safety and survival of the adoptive transfer of genetically marked syngeneic lymphocytes in HIV-infected identical twins. Hum Gene Ther. 1993;4(5):659–80.
van Lunzen J, Glaunsinger T, Stahmer I, von Baehr V, Baum C, Schilz A, et al. Transfer of autologous gene-modified T cells in HIV-infected patients with advanced immunodeficiency and drug resistant viruses. Mol Ther. 2007;15(5):1024–33.
Mitsuyasu RT, Merigan TC, Carr A, Zack JA, Winters MA, Workman C, et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15(3):285–92.
•• von Laer D, Baum C, Schambach A, Kuehlcke K, Zahn R, Newrzela S, et al. Gene therapeutic approaches for immune modulation in AIDS. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry. 2007(6):121–140. This is a comprehensive review on the current concepts of gene therapy in HIV infection. Basic research and concepts are thoroughly discussed and translated into clinical trials.
Colin L, Van Lint C. Molecular control of HIV-1 postintegration latency: implications for the development of new therapeutic strategies. Retrovirology. 2009;6:111.
Rossi JJ, June CH, Kohn DB. Genetic therapies against HIV. Nat Biotechnol. 2007;25:1444–54.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–71.
Münch J, Rücker E, Ständker L, Adermann K, Goffinet C, Schindler M, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–71.
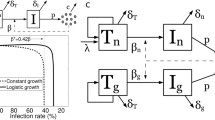
von Laer D, Hasselmann S, Hasselmann K. Impact of gene-modified T cells on HIV infection dynamics. J Theor Biol. 2006;238:60–77.
•• Hütter G, Nowak D, Mossner M, Ganepola S, Müßig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. This study demonstrated for the first time the eradication of HIV-1 in a patient successfully transplanted with allogeneic CCR5-negative hematopoietic stem cells.
Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5{Delta}32/{Delta}32 stem cell transplantation. Blood. 2010 Dec 8. [Epub ahead of print].
Friedman AD, Triezenberg SJ, McKnight SL. Expression of a truncated viral trans-activator selectively impedes lytic infection by its cognate virus. Nature. 1988;335(6189):452–4.
Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988;335(6189):395–6.
McCarthy M. Gene therapy for HIV infection. Lancet. 1993;342(8874):799.
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–9.
•• Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–58. This work has confirmed the potential of successful long-term correction of inborn severe combined immunodeficiency.
• Trobridge GD, Wu RA, Beard BC, Chiu SY, Muñoz NM, von Laer D, et al. Protection of stem cell-derived lymphocytes in a primate AIDS gene therapy model after in vivo selection. PLoS One. 2009;4(11):e7693. This is a clear demonstration that in vivo selection of hematopoietic stem cells “immunized” against HIV is possible.
Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC, et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2(8):E234.
Santoni FA, Hartley O, Luban J. Deciphering the code for retroviral integration target site selection. PLoS Comput Biol. 2010;6(11):e1001008.
Baum C, Düllmann J, Li Z, Fehse B, Meyer J, Williams DA, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–114.
• Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med. 2010;16(2):198–204. This is a detailed analysis of side effects in a gene therapy trial for chronic granulomatous disease.
Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24(6):687–96.
Modlich U, Bohne J, Schmidt M, von Kalle C, Knöss S, Schambach A, et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108(8):2545–53.
Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E, et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther. 2008;16(4):718–25.
Cornils K, Lange C, Schambach A, Brugman MH, Nowak R, Lioznov M, et al. Stem cell marking with promotor-deprived self-inactivating retroviral vectors does not lead to induced clonal imbalance. Mol Ther. 2009;17:131–43.
Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119(4):964–75. doi:10.1172/JCI37630.
• Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467(7313):318–22. This study has shown that insertional mutagenesis remains a risk factor in clinical gene therapy even with safety-optimized lentiviral vectors.
Kustikova OS, Schiedlmeier B, Brugman MH, Stahlhut M, Bartels S, Li Z, et al. Cell-intrinsic and vector-related properties cooperate to determine the incidence and consequences of insertional mutagenesis. Mol Ther. 2009;17(9):1537–47.
• Newrzela S, Cornils K, Li Z, Baum C, Brugman MH, Hartmann M, et al. Resistance of mature T cells to oncogene transformation. Blood. 2008;112:2278–86. In direct contrast with hematopoietic stem cells, polyclonal T cells were shown to be strongly resistant toward malignant transformation.
• Bohne J, Cathomen T. Genotoxicity in gene therapy: an account of vector integration and designer nucleases. Curr Opin Mol Ther. 2008;10(3):214–23. Nice overview on strategies to overcome genotoxicity of integrating gene transfer vectors.
Mátés L, Chuah MK, Belay E, Jerchow B, Manoj N, Acosta-Sanchez A, et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet. 2009;41(6):753–61.
Cornu TI, Cathomen T. Targeted genome modifications using integrase-deficient lentiviral vectors. Mol Ther. 2007;15(12):2107–13.
• Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25(11):1298–306. Epub 2007 Oct 28. This work proves the possibility to edit gene loci (as CCR5) in hematopoietic stem cells using non-integrating lentiviral vectors.
Kustikova OS, Wahlers A, Kühlcke K, Stähle B, Zander AR, Baum C, et al. Dose finding with retroviral vectors: correlation of retroviral vector copy numbers in single cells with gene transfer efficiency in a cell population. Blood. 2003;102:3934–7.
Fehse B, Roeder I. Insertional mutagenesis and clonal dominance—biological and statistical considerations. Gene Ther. 2008;15:143–53.
von Laer D, Hasselmann S, Hasselmann K. Gene therapy for HIV infection: what does it need to make it work? J Gene Med. 2006;8(6):658–67.
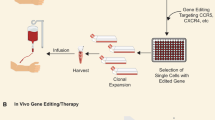
•• Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–47. This study demonstrates antiviral effects in humanized mice, which were engrafted with human hematopoietic progenitor cells. Prior to transplantation, the human cells were treated with CCR5 gene-inactivating zinc finger nuclease.
• Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–16. A study demonstrating long-lived CCR5 inhibition using zinc finger nuclease.
Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25:1298–306.
•• Sarkar I, Hauber I, Hauber J, Buchholz F. HIV-1 proviral DNA excision using an evolved recombinase. Science. 2007;316:1912–5. This study reports the quantitative excision of HIV-1 provirus from human chromosomal DNA by expression of an engineered LTR-specific recombinase.
Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–55.
Branda CS, Dymecki SM. Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28.
Disclosure
J. van Lunzen: honoraria from Vision7 GmbH; B. Fehse: stock/stock options in Vision7 GmbH; J. Hauber: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Lunzen, J., Fehse, B. & Hauber, J. Gene Therapy Strategies: Can We Eradicate HIV?. Curr HIV/AIDS Rep 8, 78–84 (2011). https://doi.org/10.1007/s11904-011-0073-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-011-0073-9