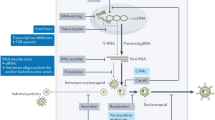
Abstract
Purpose of Review
Our purpose was to examine current and emerging biomarkers that are being utilized or under study with current therapies and examine the preliminary data with emerging therapies that will likely be combined moving forward to achieve higher rates of functional cure.
Recent Findings
Quantitative HBsAg is the most important biomarker to assess response to current and novel therapies that inhibit viral replication and restore the immune response to those with HBV infection with loss of HBsAg reflecting functional cure. Hepatitis B core-related antigen and serum HBV RNA reflect transcription from cccDNA and declines can be used to predict relapse after discontinuation of hepatitis B therapy.
Summary
Quantitative HBsAg will remain the primary biomarker to assess functional cure. There is need for an assay that measures hepatitis B-specific immune-responses to document restoration of adaptive immunity as we develop novel immunomodulatory therapies to be combined with inhibitors of the hepatitis B viral replication.

Similar content being viewed by others
References
Lim JK, Nguyen MH, Kim WR, Gish R, Perumalswami P, Jacobson IM. Prevalence of chronic hepatitis B virus infection in the United States. Am J Gastroenterol. 2020.
Ward JW, Hinman AR. What is needed to eliminate hepatitis B virus and hepatitis C virus as global health threats. Gastroenterology. 2019;156:297–310.
Liver EAFTSOTEASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;2017(67):370–98.
Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. J Hepatol. 2017;67:847–61.
Papatheodoridis G, Vlachogiannakos I, Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G, et al. Discontinuation of oral antivirals in chronic hepatitis B: a systematic review. Hepatology. 2016;63:1481–92.
Chen C, Yang HI, Su J, et al. RIsk of hepatocellular carcinoma across a biological gradient of serum hepatitis b virus dna level. JAMA. 2006;295:65–73.
Candotti D, Assennato SM, Laperche S, Allain J-P, Levicnik-Stezinar S. Multiple HBV transfusion transmissions from undetected occult infections: revising the minimal infectious dose. Gut. 2019;68:313–21.
Sulkowski MS, Agarwal K, Fung S, Yuen M, Ma X, Lalezari J, et al. Continued therapy with ABI-H0731+ NrtI results in sequential reduction/loss of HBV DNA, HBV RNA, HBeAg, HBcrAg and HBsAg in HBeAg-positive patients. National AIDS Treatment Advocacy Project. Abstr Am Assoc Study Liver Dis Annu Meet 2019.
Hong T-C, Yang H-C, Kao J-H. Role of HBsAg testing in the management of patients with chronic HBV. Current Hepatology Reports. 2019;18:331–41.
Charre C, Levrero M, Zoulim F, Scholtès C. Non-invasive biomarkers for chronic hepatitis B virus infection management. Antiviral Res. 2019;169:104553.
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83.
Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, et al. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014.
Ma H, Yang RF, Wei L. Quantitative serum HBsAg and HBeAg are strong predictors of sustained HBeAg seroconversion to pegylated interferon alfa-2b in HBeAg-positive patients. J Gastroenterol Hepatol. 2010;25:1498–506.
Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–7.
Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141–50.
Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky J-M. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676–83.
Marcellin P, Buti M, Krastev Z, Robert A, Zeuzem S, Lou L, et al. Kinetics of hepatitis B surface antigen loss in patients with HBeAg-positive chronic hepatitis B treated with tenofovir disoproxil fumarate. J Hepatol. 2014;61:1228–37.
van Bömmel F, Berg T. Stopping long-term treatment with nucleos (t) ide analogues is a favourable option for selected patients with HB eAg-negative chronic hepatitis B. Liver Int. 2018;38:90–6.
Liem KS, Fung S, Wong DK, Yim C, Noureldin S, Chen J, et al. Limited sustained response after stopping nucleos (t) ide analogues in patients with chronic hepatitis B: results from a randomised controlled trial (Toronto STOP study). Gut. 2019;68:2206–13.
Lopatin U. Drugs in the pipeline for HBV. Clin Liver Dis. 2019;23:535–55.
Zoulim F, Lenz O, Vandenbossche JJ, Talloen W, Verbinnen T, Moscalu I, et al. JNJ-56136379, an HBV capsid assembly modulator, is well-tolerated and has antiviral activity in a phase 1 study of patients with chronic infection. Gastroenterology. 2020.
Gane EJ, Locarnini S, Lim TH, Strasser S, Sievert W, Cheng W, et al. First results with rna interference (rnai) in chronic hepatitis b (chb) using ARO-HBV. Hepatology 2018; 68 (6): 1463A-1463A. Hepatology. 2018.
Yuen MF, Gane EJ, Kim DJ, Weilert F, Chan HLY, Lalezari J, et al. Antiviral activity, safety, and pharmacokinetics of capsid assembly modulator NVR 3–778 in patients with chronic HBV infection. Gastroenterology. 2019;156:1392-1403.e7.
Kimura T, Rokuhara A, Sakamoto Y, Yagi S, Tanaka E, Kiyosawa K, et al. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J Clin Microbiol. 2002;40:439–45.
Heck JA, Meng X, Frick DN. Cyclophilin B stimulates RNA synthesis by the HCV RNA dependent RNA polymerase. Biochem Pharmacol. 2009;77:1173–80.
Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615–25.
Yoshida K, Desbiolles A, Feldman SF, Ahn SH, Alidjinou EK, Atsukawa M, et al. Assay for Hepatitis B Core-related Antigen Identify Patients With High Viral Load: Systematic Review and Meta-analysis of Individual Participant Data. Clin Gastroenterol Hepatol. 2020.
van Campenhout MJ, Rijckborst V, Brouwer WP, van Oord GW, Ferenci P, Tabak F, et al. Hepatitis B core-related antigen monitoring during peginterferon alfa treatment for HBeAg-negative chronic hepatitis B. J Viral Hepatitis. 2019;26:1156–63.
Tseng T-C, Liu C-J, Hsu C-Y, Hong C-M, Su T-H, Yang W-T, et al. High level of hepatitis B core–related antigen associated with increased risk of hepatocellular carcinoma in patients with chronic HBV infection of intermediate viral load. Gastroenterology. 2019;157:1518-1529.e3.
Asahina Y, Hayashi N, Hiramatsu N, Izumi N, Koike K, Kumada H, et al. JSH guidelines for the management of hepatitis B virus infection. Hepatol Res. 2014;44:1–58.
Carey I, Gersch J, Wang B, Moigboi C, Kuhns M, Cloherty G, et al. Pregenomic HBV RNA and hepatitis B core-related antigen predict outcomes in hepatitis B e antigen–negative chronic hepatitis B patients suppressed on nucleos(T)ide analogue therapy. Hepatology. 2020;72:42–57.
Jansen L, Kootstra NA, van Dort KA, Takkenberg RB, Reesink HW, Zaaijer HL. Hepatitis B virus pregenomic RNA is present in virions in plasma and is associated with a response to pegylated interferon alfa-2a and nucleos (t) ide analogues. J Infect Dis. 2016;213:224–32.
Bai L, Zhang X, Kozlowski M, Li W, Wu M, Liu J, et al. Extracellular hepatitis B virus RNAs are heterogeneous in length and circulate as capsid-antibody complexes in addition to virions in chronic hepatitis B patients. J Virol. 2018;92.
Liu S, Zhou B, Valdes JD, Sun J, Guo H. Serum hepatitis B virus RNA: a new potential biomarker for chronic hepatitis B virus infection. Hepatology. 2019;69:1816–27.
van Bömmel F, Bartens A, Mysickova A, Hofmann J, Krüger DH, Berg T, et al. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology. 2015;61:66–76.
Farag MS, van Campenhout MJH, Pfefferkorn M, Fischer J, Deichsel D, Boonstra A, et al. Hepatitis B virus RNA as early predictor for response to pegylated interferon alpha in HBeAg-negative chronic hepatitis B. Clin Infect Dis. 2020.
Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol. 2016;65:700–10.
Yuen M-F, Agarwal K, Gane EJ, Schwabe C, Ahn SH, Kim DJ, et al. Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: a randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol Hepatol. 2020;5:152–66.
Sulkowski MS, Agarwal K, Fung S, Yuen M-F, Ma X, Lalezari J, et al. Continued therapy with ABI-H0731+ NrtI results in sequential reduction/loss of HBV DNA, HBV RNA, HBeAg, HBcrAg and HBsAg in HBeAg-positive patients. Hepatology. 2019;70:1486A-1487A.
Cornberg M, Wiegand SB. ImPortance of IP-10 in hepatitis B. Antivir Ther. 2016;21:93–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Disclosures: TRIO Network, BMS, Assembly Grant Support.
Advisory Board: Gilead, Aligos.
DSMB Janssen.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hepatitis B
Rights and permissions
About this article
Cite this article
Kwo, P.Y. Emerging Biomarkers for HBV Cure: HBsAg and Beyond. Curr Hepatology Rep 20, 151–157 (2021). https://doi.org/10.1007/s11901-021-00573-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-021-00573-x