Abstract
Purpose of Review
Malnutrition in cirrhosis is associated with significant morbidity and mortality, and an accurate energy prescription is central to effective nutrition therapy. Indirect calorimetry is recommended to determine energy requirements, with novel handheld devices offering a simpler and lower cost assessment. This review summarises the published literature from 2010 to 2020 regarding the measurement of energy expenditure in patients with liver cirrhosis.
Recent Findings
Twelve studies involving 1039 patients were identified. Results indicate discordance between predicted and measured requirements in cirrhosis, with both underestimation (58%, n = 7) and overestimation (36%, n = 4) reported. The prevalence of hypermetabolism ranged from 5.3 to 58.3%. Heterogeneity in patient cohort, liver disease severity, and nutritional status impact observed results. In three studies involving handheld calorimetry devices, moderate agreement with metabolic carts was reported, with superior results to predictive equations when determining resting metabolic rate.
Summary
Predictive equations are inaccurate in cirrhosis, and indirect calorimetry should be used to guide nutrition interventions. Handheld devices are a promising tool to embed in clinical practice with further studies required to validate their use.

Similar content being viewed by others
References
Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019;70(5):1816–29. https://doi.org/10.1002/hep.30828.
Dasarathy SMM. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–44.
Maharshi S, Sharma BC, Srivastava S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol. 2015;30(10):1507–13.
D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–31.
Harrison J, McKiernan J, Neuberger JM. A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transpl Int. 1997;10(5):369–74. https://doi.org/10.1007/s001470050072.
Lucidi CLB, Di Gregorio V, Incicco S, D'Ambrosio D, Venditti M, Riggio O, et al. A low muscle mass increases mortality in compensated cirrhotic patients with sepsis. Liver Int. 2018;38:38(5)–857. https://doi.org/10.1111/liv.13691.
Sinclair M, Chapman B, Hoermann R, Angus PW, Testro A, Scodellaro T, et al. Handgrip strength adds more prognostic value to the model for end-stage liver disease score than imaging-based measures of muscle mass in men with cirrhosis. Liver Transpl. 2019;25(10):1480–7. https://doi.org/10.1002/lt.25598.
Ebadi M, Tandon P, Moctezuma-Velazquez C, Ghosh S, Baracos VE, Mazurak VC, et al. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol. 2018;69(3):608–16. https://doi.org/10.1016/j.jhep.2018.04.015.
Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schutz T, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38(2):485–521. https://doi.org/10.1016/j.clnu.2018.12.022.
Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol. 2008;23(4):527–33. https://doi.org/10.1111/j.1440-1746.2008.05369.x.
EASL. Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–93. https://doi.org/10.1016/j.jhep.2018.06.024.
Le Cornu KA, McKierman FJ, Kaadia SA, Neuberger JM. A prospective randomised study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation. 2000;69(7):1364–9.
Campillo B, Bories PN, Pornin B, Devanlay M. Influence of liver failure, ascites, and energy expenditure on the response to oral nutrition in alcoholic liver cirrhosis. Nutrition. 1997;13(7-8):613–21. https://doi.org/10.1016/s0899-9007(97)83001-8.
Manguso F, D'Ambra G, Menchise A, Sollazzo R, D’Agostino L. Effects of an appropriate oral diet on the nutritional status of patients with HCV-related liver cirrhosis: a prospective study. Clin Nutr. 2005;24(5):751–9. https://doi.org/10.1016/j.clnu.2005.02.010.
Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48(2):557–66. https://doi.org/10.1002/hep.22367.
Swart GR, Zillikens MC, van Vuure JK, van den Berg JW. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ. 1989;299(6709):1202–3. https://doi.org/10.1136/bmj.299.6709.1202.
Kearns PJ, Young H, Garcia G, Blaschke T, O'Hanlon G, Rinki M, et al. Accelerated improvement of alcoholic liver disease with enteral nutrition. Gastroenterology. 1992;102(1):200–5. https://doi.org/10.1016/0016-5085(92)91801-a.
Cabre E, Gonzalez-Huix F, Abad-Lacruz A, Esteve M, Acero D, Fernandez-Banares F, et al. Effect of total enteral nutrition on the short-term outcome of severely malnourished cirrhotics. A randomized controlled trial. Gastroenterology. 1990;98(3):715–20. https://doi.org/10.1016/0016-5085(90)90293-a.
Ney M, Vandermeer B, van Zanten SJV, Ma MM, Gramlich L, Tandon P. Meta-analysis: oral or enteral nutrition supplementation in cirrhosis. Aliment Pharmacol Ther. 2013;37:672–279.
Koretz RL, Avenell A, Lipman TO. Nutritional support for liver disease. Cochrane Database Syst Rev. 2012;5(5):CD008344. https://doi.org/10.1002/14651858.CD008344.pub2.
Fialla AD, Israelsen M, Hamberg O, Krag A, Gluud LL. Nutritional therapy in cirrhosis or alcoholic hepatitis: a systematic review and meta-analysis. Liver Int. 2015;35(9):2072–8. https://doi.org/10.1111/liv.12798.
Reeves MM, Capra S. Predicting energy requirements in the clinical setting: are current methods evidence based? Nutr Rev. 2003;61:143–51.
Delsoglio M, Achamrah N, Berger MM, Pichard C. Indirect calorimetry in clinical practice. J Clin Med. 2019;8(9):1387. https://doi.org/10.3390/jcm8091387.
Weekes CE. Controversies in the determination of energy requirements. Proc Nutr Soc. 2007;66:367–77.
Hipskind P, Glass C, Charlton D, Nowak D, Dasarathy S. Do handheld calorimeters have a role in assessment of nutrition needs in hospitalized patients? A systematic review of literature. Nutr Clin Pract. 2011;26(4):426–33.
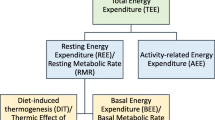
Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond). 2004;1(1):5. https://doi.org/10.1186/1743-7075-1-5.
Westerterp KR. Physical activity and physical activity induced energy expenditure in humans: measurement, determinants, and effects. Front Physiol. 2013;4:90. https://doi.org/10.3389/fphys.2013.00090.
Oshima T, Berger MM, De Waele E, Guttormsen AB, Heidegger CP, Hiesmayr M, et al. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. 2017;36(3):651–62. https://doi.org/10.1016/j.clnu.2016.06.010.
Reid CL. Poor agreement between continuous measurements of energy expenditure and routinely used prediction equations in intensive care unit patients. Clin Nutr Edinb Scotl. 2007;26:649–57.
Singer P, Singer J. Clinical guide for the use of metabolic carts: indirect calorimetry: no longer the orphan of energy estimation. Nutr Clin Pract. 2016;31(1):30–8.
Eslamparast T, Vandermeer B, Raman M, Gramlich L, Den Heyer V, Belland D, et al. Are predictive energy expenditure equations accurate in cirrhosis? Nutrients. 2019;11(2):334. https://doi.org/10.3390/nu11020334.
Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institute; 1919.
University FaAOWHOUN. Energy and protein requirements. Report of a joint FAO/WHO/UNU expert consultation. WHO Technical Report Series. 1985:724.
Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. https://doi.org/10.1093/ajcn/51.2.241.
Owen OE, Kavle E, Owen RS, Polansky M, Caprio S, Mozzoli MA, et al. A reappraisal of caloric requirements in healthy women. Am J Clin Nutr. 1986;44(1):1–19. https://doi.org/10.1093/ajcn/44.1.1.
Owen OE, Holup JL, D'Alessio DA, Craig ES, Polansky M, Smalley KJ, et al. A reappraisal of the caloric requirements of men. Am J Clin Nutr. 1987;46(6):875–85. https://doi.org/10.1093/ajcn/46.6.875.
Cunningham JJ. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr. 1980;33(11):2372–4. https://doi.org/10.1093/ajcn/33.11.2372.
Muller MJ, Bottcher J, Selberg O. Energy expenditure and substrate metabolism in liver cirrhosis. Int J Obes Relat Metab Disord. 1993;17(Suppl 3):S102–6.
Dolz C, Raurich JM, Ibanez J, Obrador A, Marse P, Gaya J. Ascites increases the resting energy expenditure in liver cirrhosis. Gastroenterology. 1991;100(3):738–44. https://doi.org/10.1016/0016-5085(91)80019-6.
Knudsen AW, Krag A, Nordgaard-Lassen I, Frandsen E, Tofteng F, Mortensen C, et al. Effect of paracentesis on metabolic activity in patients with advanced cirrhosis and ascites. Scand J Gastroenterol. 2016;51(5):601–9. https://doi.org/10.3109/00365521.2015.1124282.
Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85(5):1257–66. https://doi.org/10.1093/ajcn/85.5.1257.
Schock L, Lam L, Tandon P, Taylor L, Raman M. Indirect calorimetry performance using a handheld device compared to the metabolic cart in outpatients with cirrhosis. Nutrients. 2019;11(5):1030. https://doi.org/10.3390/nu11051030.
Glass C, Hipskind P, Cole D, Lopez R, Dasarathy S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: a prospective study. Nutr Clin Pract. 2012;27:677–88.
Terakura Y, Shiraki M, Nishimura K, Iwasa J, Nagaki M, Moriwaki H. Indirect calorimetry and anthropometry to estimate energy metabolism in patients with liver cirrhosis. J Nutr Sci Vitaminol (Tokyo). 2010;56(6):372–9. https://doi.org/10.3177/jnsv.56.372.
Merli M, Riggio O, Romiti A, Ariosto F, Mango L, Pinto G, et al. Basal energy production rate and substrate use in stable cirrhotic patients. Hepatology. 1990;12(1):106–12. https://doi.org/10.1002/hep.1840120117.
Campillo B, Richardnet JP, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003;19(6):515–21.
Prieto-Frias C, Conchillo M, Payeras M, Inarrairaegui M, Davola D, Fruhbeck G, et al. Factors related to increased resting energy expenditure in men with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28(2):139–45. https://doi.org/10.1097/MEG.0000000000000516.
Meng QH, Hou W, Yu HW, Lu J, Li J, Wang JH, et al. Resting energy expenditure and substrate metabolism in patients with acute-on-chronic hepatitis B liver failure. J Clin Gastroenterol. 2011;45(5):456–61. https://doi.org/10.1097/MCG.0b013e31820f7f02.
Shiraki M, Terakura Y, Iwasa J, Shimizu M, Miwa Y, Murakami N, et al. Elevated serum tumor necrosis factor-alpha and soluble tumor necrosis factor receptors correlate with aberrant energy metabolism in liver cirrhosis. Nutrition. 2010;26(3):269–75. https://doi.org/10.1016/j.nut.2009.04.016.
Schutz T, Hudjetz H, Roske AE, Katzorke C, Kreymann G, Budde K, et al. Weight gain in long-term survivors of kidney or liver transplantation—another paradigm of sarcopenic obesity? Nutrition. 2012;28(4):378–83. https://doi.org/10.1016/j.nut.2011.07.019.
Miyake R, Tanaka S, Ohkawara K, Ishikawa-Takata K, Hikihara Y, Taguri E, et al. Validity of predictive equations for basal metabolic rate in Japanese adults. J Nutr Sci Vitaminol (Tokyo). 2011;57(3):224–32. https://doi.org/10.3177/jnsv.57.224.
Mathur S, Peng S, Gane EJ, McCall JL, Plank LD. Hypermetabolism predicts reduced transplant-free survival independent of MELD and Child-Pugh scores in liver cirrhosis. Nutrition. 2007;23(5):398–403. https://doi.org/10.1016/j.nut.2007.02.003.
Ferreira LG, Santos LF, Silva TR, Anastacio LR, Lima AS, Correia MI. Hyper- and hypometabolism are not related to nutritional status of patients on the waiting list for liver transplantation. Clin Nutr. 2014;33(5):754–60. https://doi.org/10.1016/j.clnu.2013.10.016.
Teramoto A, Yamanaka-Okumura H, Urano E, Nakamura-Kutsuzawa T, Sugihara K, Katayama T, et al. Comparison of measured and predicted energy expenditure in patients with liver cirrhosis. Asia Pac J Clin Nutr. 2014;23(2):197–204. https://doi.org/10.6133/apjcn.2014.23.2.12.
Messina C, Maffi G, Vitale J, Ulivieri F, Guglielmi G, Sconfienza L. Diagnostic imaging of osteoporosis and sarcopenia: a narrative review. Quant Imaging Med Surg. 2018;8:86–99.
Kato M, Miwa Y, Tajika M, Hiraoka T, Muto Y, Moriwaki H. Preferential use of branched-chain amino acids as an energy substrate in patients with liver cirrhosis. Intern Med. 1998;37:429–34.
Belarmino G, Singer P, Gonzalez MC, Machado NM, Cardinelli CS, Barcelos S, et al. Prognostic value of energy expenditure and respiratory quotient measuring in patients with liver cirrhosis. Clin Nutr. 2019;38:1899–904.
Lee SJ, Lee HJ, Jung YJ, Han M, Lee SG, Hong SK. Comparison of measured energy expenditure using indirect calorimetry vs predictive equations for liver transplant recipients. JPEN J Parenter Enteral Nutr. 2020;45:761–7. https://doi.org/10.1002/jpen.1932.
Case KO, Brahler CJ, Heiss C. Resting energy expenditures in Asian women measured by indirect calorimetry are lower than expenditures calculated from prediction equations. J Am Diet Assoc. 1997;97(11):1288–92. https://doi.org/10.1016/s0002-8223(97)00308-8.
Bot D, Droop A, Tushuizen ME, Van Hoek B. For dietary advice in end-stage liver cirrhosis resting metabolic rate should be measured, not estimated. Hepatoma Research. 2020;45. https://doi.org/10.20517/2394-5079.2020.62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Brooke Chapman, Adam Testro, Paul Gow, Bethany Whitcher, and Maria Sinclair declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chapman, B., Testro, A., Gow, P. et al. Determining Energy Requirements in Cirrhosis: an Update on the Role of Indirect Calorimetry. Curr Hepatology Rep 20, 85–95 (2021). https://doi.org/10.1007/s11901-021-00564-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-021-00564-y