Abstract
Purpose of Review
Chronic hepatitis B (CHB), caused by hepatitis B virus (HBV), is a major cause of advanced liver disease and hepatocellular carcinoma (HCC) worldwide. HBV replication is characterized by the synthesis of covalently closed circular (ccc) DNA which is not targeted by antiviral nucleos(t)ide analogues (NUCs) the key modality of standard of care. While HBV replication is successfully suppressed in treated patients, they remain at risk for developing HCC. While functional cure, characterized by loss of HBsAg, is the first goal of novel antiviral therapies, curative treatments eliminating cccDNA remain the ultimate goal. This review summarizes recent advances in the discovery and development of novel therapeutic strategies and their impact on cccDNA biology.
Recent Findings
Within the last decade, substantial progress has been made in the understanding of cccDNA biology including the discovery of host dependency factors, epigenetic regulation of cccDNA transcription and immune-mediated degradation. Several approaches targeting cccDNA either in a direct or indirect manner are currently at the stage of discovery, preclinical or early clinical development. Examples include genome-editing approaches, strategies targeting host dependency factors or epigenetic gene regulation, nucleocapsid modulators and immune-mediated degradation.
Summary
While direct-targeting cccDNA strategies are still largely at the preclinical stage of development, capsid assembly modulators and immune-based approaches have reached the clinical phase. Clinical trials are ongoing to assess their efficacy and safety in patients including their impact on viral cccDNA. Combination therapies provide additional opportunities to overcome current limitations of individual approaches.
Similar content being viewed by others
Introduction
Chronic hepatitis B (CHB), caused by hepatitis B virus (HBV), is a worldwide leading cause of liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC) [1, 2]. HCC is currently the second cause of death from cancer, and more than 50% of HCC cases are related to HBV infection in the most affected geographic areas [2, 3•]. Despite the availability of an effective prophylactic vaccine, HBV remains a worldwide major public health concern with an estimated prevalence of 250–300 million infected people globally [4••].
Current treatments against CHB include nucleos(t)ide analogues (NUCs) (such as lamivudine or entecavir) and pegylated interferon-α (PEG-IFN-α) [5, 6]. NUCs have been proven to effectively control HBV infection by suppression of viral replication and thus improve quality of life and survival in patients; however, they are not curative. Furthermore, NUCs are generally long-term or lifelong treatments. PEG-IFN-α-based therapies can result in viral cure in a small subset of patients; however, these therapies are limited by a low response rate and significant side effects, which preclude a widespread application of this strategy [7]. Sustained viral replication and liver injury are recognized as key risk factors for HBV-related HCC, which are reduced by antiviral therapy [5, 6]. Nevertheless, successful viral control in treated patients reduces but does not eliminate the risk of HCC, whose annual incidences range from 0.9 to 5.4% under treatment with NUCs in the presence of cirrhosis [8]. Most approaches in clinical development aim for functional cure characterized by sustained loss of hepatitis B surface antigen with or without hepatitis B surface antibody seroconversion, which is associated with improved clinical outcomes [9, 10, 13]. Since persistent cccDNA acts as a reservoir for viral relapse [11••], eradication of HBV DNA including intrahepatic covalently closed circular DNA (cccDNA), i.e. complete sterilizing cure, is the ultimate goal [10, 12, 13]. However, sterilizing cure is much more difficult to achieve [10, 12, 13].
HBV infection into human hepatocytes is thought to be initiated by binding to heparan sulphate proteoglycans including glypican 5 (GPC5) [14••] with subsequent viral cell entry mediated by the sodium taurocholate cotransporting polypeptide (NTCP) with EGF receptor (EGFR) as a facilitator [15••, 16••, 17••]. The genome of HBV is a 3.2 kb relaxed circular (rc) DNA whose one of the two strands is covalently linked to the viral polymerase. Upon infection, the viral genome is translocated and released into the host cell nucleus where the rcDNA is converted into an episomal cccDNA. cccDNA is the transcriptional template for all viral gene products, including the pregenomic RNA (pgRNA). The pgRNA is selectively packaged into a capsid and then reverse transcribed into rcDNA. Mature nucleocapsid can be used for cccDNA amplification or be enveloped to release a generation of new virions [18•].
In the nucleus of infected hepatocytes, cccDNA persists as a stable minichromosome [19, 20] associated with histone and non-histone proteins [21]. Gene expression is regulated by cellular factors including transcription factors and chromatin-modifying enzymes [22]. Viral proteins HBc (core) and HBx support the structural and functional features of cccDNA [23]. It is noteworthy that a few cccDNA copies per liver cell are sufficient to reactivate viral multiplication after therapy withdrawal or loss of immunological control. Therefore, maintenance of cccDNA with ineffective immune responses leads to HBV chronicity [23].
Thus, the ideal therapeutic strategy for curative approaches includes reduction or elimination of the whole cccDNA pool. Addressing this issue, we review here how novel therapeutic strategies affect cccDNA in a direct or indirect manner including genome editing, epigenetics and gene regulation, host-targeting approaches, nucleocapsid assembly and immunity.
Direct cccDNA Degradation
The most direct anti-cccDNA strategy is its specific degradation, whose long-standing difficulty was recently overcome by new genome-editing technologies [24] (Fig. 1). The major editing systems, including the zinc finger nuclease (ZFN) [25], transcription activator-like effector nucleases (TALENs) [26] and the clustered regularly interspaced short palindromic repeats/CRISPR-associated (CRISPR/Cas) system [27], have been used to disrupt HBV cccDNA. All these editing systems create a DNA double-strand break in a specific target site and repair the cleavage sites by altering DNA sequence. Particularly, the CRISPR/Cas9 system was the most successful [28], arousing widespread interest according to its simplicity and flexibility [29]. CRISPR/Cas9-based anti-HBV effects including suppression of cccDNA were increasingly reported and thoroughly summarized elsewhere [30, 31]. Reduction of intrahepatic cccDNA was validated in vivo, consistent with the cell culture results [32]. Meanwhile, several challenges remain [33], primarily represented by off-target effects and delivery. Indeed, off-target insertions and deletions (indels) were associated with CRISPR/Cas9-mediated HBV genome inactivation [34]. Then, these editing systems have adverse events, both in terms of non-specific cleavage of host genome and integrated HBV genome [35]. In recent years, Cas9 variants, engineered Cas9 and modified guide RNAs were demonstrated to improve the specificity of the CRISPR/Cas9 system [36], and hitherto Cas9 nickases with less off-target effects were successfully harnessed against HBV [37,38,39]. Delivery also poses challenges, and various methods have been examined encompassing viruses, cation lipids, nanoparticles and nanomolecular DNA traps to address this limitation [40]. Notably the smaller version of Cas9 protein from Staphylococcus aureus, SaCas9, seems adapted for delivery by adeno-associated virus (AAV)-mediated delivery [41•], leading to cccDNA inactivation in vivo [42, 43]. Alternatively, small molecules that directly act on cccDNA are currently investigated. In this respect, an HBV cccDNA destabilizer (termed ccc_R08) resulted in reduced cccDNA levels in HBV-infected hepatocytes and in the liver of treated mice [44]. The nature of the molecule and its mode of action have not been disclosed yet. A second small molecule, CCC-0975, was able to reduce cccDNA biosynthesis [45]. While conceptually highly innovative, the approaches described above are largely still at the discovery or preclinical stage of development.
Therapeutic strategies and their potential impact on viral cccDNA within the HBV life cycle. Upon infection, the viral genome is translocated and released into the nucleus where the rcDNA is converted into an episomal covalently closed circular DNA (cccDNA). CHB is linked to the persistence of the cccDNA, and a few cccDNA copies per liver cell can reactivate full virus production after therapy withdrawal. Chronic hepatitis B cure is believed to require cccDNA elimination or functional knockout of cccDNA by silencing of cccDNA activity. Examples for strategies aiming for HBV cure include (1) elimination of cccDNA by gene editing, (2) prevention of cccDNA accumulation by blocking host factors involved in cccDNA formation, (3) silencing of cccDNA transcription by targeting epigenetic regulation, (4) nucleocapsid assembly modulators by preventing reimport of newly synthetized nucleocapsid and thereby prevent amplification of the cccDNA pool, preventing formation of cccDNA and maybe playing a role in the cccDNA structure itself and (5) immune-mediated clearance of the cccDNA or cccDNA expressing hepatocytes
Targeting Host Factors Required for cccDNA Formation and Biology
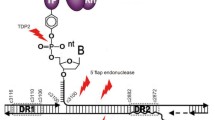
Instead of directly targeting the cccDNA, another approach is to target the host dependency factors for cccDNA formation or maintenance. Notably, cellular factors involved in the DNA repair machinery are expected to play a key role in the rcDNA-to-cccDNA conversion, and the recent identification of such key factors involved in cccDNA formation may provide promising opportunities for the development of new antivirals for HBV cure [5, 46, 47] (Fig. 1). First, an elegant study identified tyrosyl-DNA-phosphodiesterase 2 (TDP2) as a crucial factor for polymerase release from the HBV rcDNA [48•]. Through the use of multiple polymerase screening, Qi and collaborators identified the DNA polymerase K (POLK), a gamma-family DNA polymerase, as a key factor in the completion of the positive strand during DP-rcDNA conversion into cccDNA [49]. In addition, the cellular pre-mRNA processing factor 31 (PRPF31) was shown to be involved in cccDNA formation or maintenance [50]. PRPF31 interacted with HBx in the nucleus which enhanced cccDNA formation. Flap endonuclease 1 protein was shown to bind and cleave the 5′-flap structure of HBV rcDNA in vitro in order to promote cccDNA formation [51]. Furthermore, DNA ligases have been shown to be crucial for cccDNA formation [52]. By using multiple specific inhibitors and siRNA, the cellular DNA damage repair ATR-CHK1 pathway was shown to be involved in rcDNA processing and HBV cccDNA formation. Once activated, the ATR-CHK1 pathway can recruit many host DNA repair factors which are likely play a key role in cccDNA formation from rcDNA [53]. Finally, Wei and Ploss identified five core components of lagging-strand synthesis as essential to cccDNA formation: proliferating cell nuclear antigen, the replication factor C complex, DNA polymerase δ, flap endonuclease 1 and DNA ligase 1. In this study, the authors suggested that these components represent the minimal set of factors required for cccDNA formation [54•].
The redundancy of the DNA repair factors however suggests the involvement of other host factors involved in cccDNA formation and regulation [55]. Their identification is a step toward the molecular understanding of HBV persistence and toward identification of new antiviral targets. Host-targeting antivirals have emerged as a promising approach for the treatment of viral infections, and some are clinically available, such as the chemokine receptor type 5 antagonist maraviroc for human immunodeficiency virus (HIV) treatment [56]. However, since HBV host dependency factors are also involved in the gene regulation of the host, adverse effects need to be carefully assessed [46]. These approaches are currently at the discovery stage of development.
Silencing of cccDNA Transcription
Another therapeutic approach is the disruption of cccDNA function with silencing of viral gene transcription through the modulation of epigenetic modifications influencing cccDNA formation and control of its transcription [28, 57] (Fig. 1). Targeting epigenetic modulators is a promising approach that has already been developed and approved for cancer treatment and has been investigated against HIV and Epstein-Barr virus (EBV) infections [58, 59].
The cccDNA is organized as a minichromosome with histone and non-histone proteins and bears binding sites for various transcription factors [21, 60]. These factors include the hepatocyte nuclear factor (HNF1, HNF3, HNF4), the retinoid X receptor (RXR) and the CCAAT-enhancer-binding protein (C/EBP). Viral proteins HBx and HBc are also involved in cccDNA activity [61]. Indeed, the regulatory protein HBx is necessary for HBV transcription from cccDNA [62]. The Smc5/6 complex has been identified as an HBx interacting partner and HBV restriction factor playing a functional role in gene expression [63]. Furthermore, interferon-α (IFN-α) has been shown to reduce cccDNA-mediated transcription of viral RNA and decrease cccDNA-bound histone acetylation such as H3K9 and H3K26 marks [64, 65]. Interleukin-6 (IL-6) represses HBV replication by decreasing cccDNA-bound histone acetylation and HNF4 expression [66, 67]. The HBV genome contains three predicted CpG islands. DNA methylation on CpG islands is catalysed by DNA methyltransferases (DNMTs) in mammalian cells and generally associated with transcription silencing [68]. The involvement of DNMTs in the HBV life cycle has not been clearly elucidated yet, although methylation of CpG islands into HBV genome leads to a decreased pgRNA expression and HBV replication [69]. Targeting DNMTs could consequently constitute a strategy to inhibit cccDNA activity.
On the other hand, histone modifications such as acetylation and methylation of cccDNA-bound H3 and H4 affect cccDNA-mediated transcription. Notably, hyperacetylation and hypoacetylation of cccDNA-bound H3 and H4 histones lead to an increased and decreased HBV replication in HBV-infected patients, respectively [70•]. Furthermore, histone deacetylase 11 (HDAC11) modulates the transcription activity of cccDNA without affecting its formation by specifically decreasing the acetylation level of histone H3 [71]. Moreover, the histone deacetylase SIRT2 inhibitor AGK2 blocks cccDNA transcription in vitro and in vivo providing another therapeutic target [72]. Another study has shown that the inhibition of the HDAC KDM5 increases the H3K4Me3 and inhibits the HBV replication [73]. Finally, non-coding RNA such as microRNA (miRNA) can target and influence HBV replication by binding to HBV mRNA or by targeting host factors. For instance, microRNA-1 (miR-1) was shown to increase HBV transcription by targeting HDAC4 and E2F transcription factor 5 [74]. Long non-coding RNA (lncRNA) PCNAP1 promotes HBV replication and cccDNA accumulation by modulating miR-154/PCNA/HBV cccDNA pathway [75].
Collectively, these findings demonstrate that gene regulation of cccDNA represents an alternative therapeutic option to target cccDNA for HBV cure. However, the limitations of these strategies are the potential adverse of the different molecules and their inability to efficiently eliminate the cccDNA pool in human hepatocytes [76], thus requiring most likely long-term treatment.
Nucleocapsid Assembly Modulators
A well-studied target of intervention is the HBV core protein (HBc) that regulates many processes in the viral life cycle such as capsid assembly, reverse transcription and virion secretion [77]. Therefore, capsid assembly modulators (CAMs) [4] (Fig. 1) have been developed to disrupt the functional roles of HBc, thereby deterring cccDNA formation. CAMs affect cccDNA levels by several mechanisms: (1) prevent reimport of newly synthetized nucleocapsid and thereby prevent amplification of the cccDNA pool [78, 79], (2) prevent formation of cccDNA in newly infected cells (probably by preventing nuclear capsid import) [80] and (3) possibly play a role in the cccDNA structure itself (since HBc is associated to cccDNA). For instance, HBc associated with CpG island 2 of cccDNA increased serum HBV DNA levels in CHB patients [81] while potentially recruiting APOBEC3A to cccDNA for its degradation [82].
Various CAMs have been developed and categorized into two major types. The first one, represented by heteroaryldihydropyrimidines (HAPs), misdirects capsid assembly (CAM-A where A stands for aberrant since these CAMs induce empty capsids with an aberrant structures), and the second one represented by phenylpropenamides (PPAs) and sulfamoylbenzamides (SBAs) induces the assembly of empty capsids (CAM-N where N stands for normal since these CAMs induce empty capsids with a normal appearance) [83]. HAPs [84,85,86], PPAs [87,88,89] as well as SBAs [90] demonstrated antiviral activities and sustained suppression of HBV DNA levels in vivo. Moreover, formation of cccDNA was shown to be inhibited by JNJ-6379 in vitro [91]. Representative HAPs (Bay 41–4109 and GLS4) and a SBA ENAN-34017 [80] also inhibited de novo synthesis of cccDNA. In recent years, discovery of new CAMs has markedly accelerated [83, 92,93,94]. Indeed, CAMs have been shown to disrupt amplification and formation of cccDNA [95, 96]. Several CAMs are in clinical development including JNJ-56136379 [97], JNJ-6379 [98], ABI-H0731 [99] and NVR 3–778 [100]. Long-term studies are under way to assess viral resistance and long-term virological response including cccDNA elimination.
Immune-Mediated Degradation of cccDNA
Another appealing approach is the immune-mediated degradation of the cccDNA [28]. An interplay of innate and adaptive immunity responses is essential for viral clearance comprising both non-cytolytic and cytolytic clearance [12••]. The concept of harnessing the patient’s immune system is supported by the fact that interferon-based therapies can result in a sustained virologic response with HBsAg loss and the elimination of the cccDNA [9]. However, IFN-based therapies are limited by significant side effects, and therefore, only few patients are successfully treated. Thus, complementary approaches exploiting antiviral immune-mediated pathways are being developed [101].
In HBV-infected chimpanzees, viral clearance with reduction of cccDNA is observed in a non-cytolytic fashion [102]. Cytokines secreted by immune cells have been suggested to control non-cytolytic viral clearance [102, 103]. Intracellular pattern recognition receptors (PRRs) like toll-like receptors (TLRs) initiate the immune responses by inducting the production of antiviral cytokines and mediators such as IFNs and by inducing the activation of natural killer and T cells [104, 105]. In mouse models, HBV replication can be reduced by TLR activation. TLR3, 7/8 and 9 recognize endosomal viral nucleic acids and induce a type 1 IFN response [106]. While the TLR7 agonist GS-9620 has shown antiviral efficacy in cell culture models [107], a reduction in viremia and HBsAg in chimpanzees [108] and decrease in cccDNA levels in the woodchuck model [109], it did not result in robust antiviral effects in humans [104, 110]. Additional TLR7 agonists such as RO7020531 and JNJ-4964 are currently evaluated for CHB in clinical trials [111]. The TLR8 agonist GS-9688 substantially reduced viral DNA, RNA, antigen and cccDNA levels in the woodchuck model [111] and is now in clinical development [111]. A recent study identified IL-2 as a potential immunotherapeutic strategy able to rescue CD8+ T cells rendered dysfunctional by hepatocellular tumour initiation [112]. In addition, TRL2 was downregulated in hepatocytes, Kupffer cells and peripheral blood mononuclear cells from CHB patients [113] with an impairment of cytokine production [114]. Conversely, an increase in TLR2-positive monocytes was associated with a better response to PEG-IFN-α treatment [115•] and enhanced TLR2 expression on monocytes led to IL-6 production [116•].In cell culture, a TLR2 ligand showed a strong anti-HBV activity [117]. Pam3CSK4 and TLR3 ligands are also efficient and may decease level of cccDNA [118]. Otherwise, degradation of cccDNA was found to be induced by apolipoprotein B mRNA editing enzyme catalytic polypeptide (APOBEC) 3 proteins. IFN-α, IFN-g, TNF-a as well as agonization of the lymphotoxin-β receptor upregulated APOBEC3A and APOBEC3B, deaminating cccDNA for its degradation [82, 102, 103]. Interestingly, overexpression of the cGAS-STING pathway reduced viral cccDNA levels in cell culture models [119]. A RIG-I agonist (inarigivir also termed SB 9200) elicited reduction in viral DNA, RNA, antigens and cccDNA in woodchucks [120] and decreased viral DNA and RNA in CHB patients [111]. However, the overall antiviral efficacy of these approaches has been limited most likely precluding their application as monotherapy.
Broad, sustained and robust antiviral T cell responses are well known to play a key role in viral cure with elimination of cccDNA in spontaneous self-limited HBV infection [121]. Thus, several strategies to improve impaired HBV-specific T cell responses in chronic HBV patients are being explored [122, 123]. Examples include therapeutic vaccines [124] and check point inhibitors (anti-programmed cell death-1 (PD-1), anti-CTLA-4) [125]. Therapeutic vaccines aim to induce or improve impaired or absent antiviral T cell responses in patient with CHB [124]. Approaches using the HBsAg-based prophylactic or vaccines with multiple viral antigens showed limited efficacy in humans [126••]. Therapeutic vaccines based on additional antigens and vectors such as modified vaccinia viruses or adenovirus have been developed and undergo clinical evaluation including prime-boost strategies [124, 127, 128]. Moreover, clinical trials for HBV viral vector vaccines and adjuvant protein vaccine (GSK3528869A) have been started (NCT03866187). While conceptually appealing, the clinical efficacy of therapeutic vaccination remains to be determined.
Immune check point proteins such as PD-1 are targeted for restoration of anti-HBV immune response breaking immune tolerance. Preclinically, the combination of an antibody to the PD-1 ligand (programmed death ligand 1) [129] with DNA vaccination resulted in complete viral clearance in a woodchuck model [130], successfully supporting the therapeutic potential of restored T cell response [131]. Indeed, blockade of PD-1 has been shown to partially restore HBV-specific T cell function [126••]. Clinical trials, e. g. for anti-PD-1 mAb nivolumab for HBV, are underway [126••]. A key challenge is an overstimulation of the immune system with severe adverse effects such as autoimmunity [125]. Moreover, reactivation of HBV infection has been observed under check point therapy [132]. While clinical studies in HBV-infected patients so far exhibited general tolerability, a risk of potentially very severe or lethal adverse effects is a clear limitation [133, 134]. Further studies are needed to understand the role of check point inhibitors in the management of CHB.
Conclusions
Most of the current approaches aim for functional cure characterized by HBsAg loss. The ultimate goal of sterilizing cure with loss of cccDNA is desirable but much more difficult to achieve. In that regard, substantial progress has been made in the understanding of the cccDNA biology such as the identification of novel host dependency factors and previously unknown mechanisms of epigenetic regulation of cccDNA transcription. Several strategies directly or indirectly targeting cccDNA are in preclinical or early clinical development (examples shown in Table 1). Given the complexity of the HBV life cycle, it is likely that combination therapies, e. g. a combination of direct-acting antiviral(s) and immune-targeting approach(es), will be required for HBV cure including the elimination of HBV cccDNA. Further studies are needed to understand and assess the efficacy and safety of the therapeutic strategies in clinical trials. The finding that patients can spontaneously eliminate HBV infection suggests that the development of curative therapies is an achievable goal. A more detailed understanding of these mechanisms in patients may provide additional opportunities for curative therapies including elimination of cccDNA.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4:135–84.
Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet Lond Engl. 2014;384:2053–63.
• Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G (2016) Hepatocellular carcinoma. Nat Rev Dis Primer 2:16018. Important review on liver cancer and hepatocellular carcinoma.
•• Schinazi RF, Ehteshami M, Bassit L, Asselah T (2018) Towards HBV curative therapies. Liver Int Off J Int Assoc Study Liver 38 Suppl 1:102–114. Important review summarizing recent therapeutic strategies that are currently being evaluated at the preclinical and clinical stage.
Liang TJ, Block TM, McMahon BJ, Ghany MG, Urban S, Guo J-T, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatol Baltim Md. 2015;62:1893–908.
Loomba R, Liang TJ. Treatment of chronic hepatitis B. Antivir Ther. 2007;12(Suppl 3):H33–41.
Ghany MG. Current treatment guidelines of chronic hepatitis B: the role of nucleos(t)ide analogues and peginterferon. Best Pract Res Clin Gastroenterol. 2017;31:299–309.
Colombo M, Lleo A. The impact of antiviral therapy on hepatocellular carcinoma epidemiology. Hepatic Oncol. 2018;5:HEP03.
Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018;319:1802–13.
Do A, Reau NS (2020) Chronic viral hepatitis: current management and future directions. hepatol commun 4:329–341.
•• Nassal M (2015) HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 64:1972–1984. Important review summarizing current knowledge on cccDNA molecular biology and potentially curative therapies.
•• Revill PA, Chisari FV, Block JM, et al (2019) A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol 4:545–558. Important review summarizing the strategy to cure HBV.
Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. J Hepatol. 2017;67:847–61.
•• Verrier ER, Colpitts CC, Bach C, et al (2016) A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatol Baltim Md 63:35–48. This study identified GPC5 as an entry factor for HBV and HDV.
•• Yan H, Zhong G, Xu G, et al (2012) Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1:e00049. This study identified NTCP as an entry factor for HBV and HDV.
•• Ni Y, Lempp FA, Mehrle S, et al (2014) Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146:1070-1083.e6. Human NTCP is a specific receptor for HBV and HDV.
Iwamoto M, Saso W, Sugiyama R, Ishii K, Ohki M, Nagamori S, et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc Natl Acad Sci U S A. 2019;116:8487–92.
• Beck J, Nassal M (2007) Hepatitis B virus replication. World J Gastroenterol 13:48–64. Important review summarizing the HBV replication.
Newbold JE, Xin H, Tencza M, Sherman G, Dean J, Bowden S, et al. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J Virol. 1995;69:3350–7.
Bock CT, Schranz P, Schröder CH, Zentgraf H. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes. 1994;8:215–29.
Bock CT, Schwinn S, Locarnini S, Fyfe J, Manns MP, Trautwein C, et al. Structural organization of the hepatitis B virus minichromosome. J Mol Biol. 2001;307:183–96.
Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51:581–92.
Allweiss L, Dandri M (2017) The role of cccDNA in HBV maintenance. Viruses. 21;9(6):156.
Carroll D. Genome editing: past, present, and future. Yale J Biol Med. 2017;90:653–9.
Ghany MG, Block TM. Disease pathways and mechanisms of potential drug targets. Clin Liver Dis. 2018;12:12–8.
Bloom K, Maepa MB, Ely A, Arbuthnot P (2018) Gene therapy for chronic HBV-can we eliminate cccDNA? Genes. 12;9(4):207.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Durantel D, Zoulim F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J Hepatol. 2016;64:S117–31.
Zhu A, Liao X, Li S, Zhao H, Chen L, Xu M, et al. HBV cccDNA and its potential as a therapeutic target. J Clin Transl Hepatol. 2019;7:258–62.
Lee C (2019) CRISPR/Cas9-based antiviral strategy: current status and the potential challenge. Mol Basel Switz. 5;24(7):1349.
Ramanan V, Shlomai A, Cox DBT, Schwartz RE, Michailidis E, Bhatta A, et al. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci Rep. 2015;5:10833.
Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antivir Res. 2015;118:110–7.
El-Kenawy A, Benarba B, Neves AF, de Araujo TG, Tan BL, Gouri A. Gene surgery: potential applications for human diseases. EXCLI J. 2019;18:908–30.
Li H, Sheng C, Wang S, et al. Removal of integrated hepatitis B virus DNA using CRISPR-Cas9. Front Cell Infect Microbiol. 2017;7:91.
Schinazi RF, Ehteshami M, Bassit L, Asselah T. Towards HBV curative therapies. Liver Int Off J Int Assoc Study Liver. 2018;38(Suppl 1):102–14.
Li J, Hong S, Chen W, Zuo E, Yang H. Advances in detecting and reducing off-target effects generated by CRISPR-mediated genome editing. J Genet Genomics Yi Chuan Xue Bao. 2019;46:513–21.
Karimova M, Beschorner N, Dammermann W, Chemnitz J, Indenbirken D, Bockmann JH, et al. CRISPR/Cas9 nickase-mediated disruption of hepatitis B virus open reading frame S and X. Sci Rep. 2015;5:13734.
Song J, Zhang X, Ge Q, Yuan C, Chu L, Liang H-F, et al. CRISPR/Cas9-mediated knockout of HBsAg inhibits proliferation and tumorigenicity of HBV-positive hepatocellular carcinoma cells. J Cell Biochem. 2018;119:8419–31.
Sakuma T, Masaki K, Abe-Chayama H, Mochida K, Yamamoto T, Chayama K. Highly multiplexed CRISPR-Cas9-nuclease and Cas9-nickase vectors for inactivation of hepatitis B virus. Genes Cells Devoted Mol Cell Mech. 2016;21:1253–62.
Biagioni A, Laurenzana A, Margheri F, Chillà A, Fibbi G, Del Rosso M. Delivery systems of CRISPR/Cas9-based cancer gene therapy. J Biol Eng. 2018;12:33.
• Ran FA, Cong L, Yan WX, et al (2015) In vivo genome editing using Staphylococcus aureus Cas9. Nature 520:186–191. Study demonstrating that SaCas9 can mediate genome editing in vivo with high specificity.
Liu Y, Zhao M, Gong M, Xu Y, Xie C, Deng H, et al. Inhibition of hepatitis B virus replication via HBV DNA cleavage by Cas9 from Staphylococcus aureus. Antivir Res. 2018;152:58–67.
Scott T, Moyo B, Nicholson S, Maepa MB, Watashi K, Ely A, Weinberg MS, Arbuthnot P (2017) ssAAVs containing cassettes encoding SaCas9 and guides targeting hepatitis B virus inactivate replication of the virus in cultured cells. Sci Rep 7:7401.
Wang L, Zhu Q, Zeng J, Yan Z, Feng A, Young J, et al. PS-074-A first-in-class orally available HBV cccDNA destabilizer ccc_R08 achieved sustainable HBsAg and HBV DNA suppression in the HBV circle mouse model through elimination of cccDNA-like molecules in the mouse liver. J Hepatol. 2019;70:e48.
Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, et al. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56:4277–88.
Baumert TF, Verrier ER, Nassal M, Chung RT, Zeisel MB. Host-targeting agents for treatment of hepatitis B virus infection. Curr Opin Virol. 2015;14:41–6.
Mohd-Ismail NK, Lim Z, Gunaratne J, Tan Y-J (2019) Mapping the interactions of HBV cccDNA with host factors. Int J Mol Sci. 1;20(17):4276.
• Königer C, Wingert I, Marsmann M, Rösler C, Beck J, Nassal M (2014) Involvement of the host DNA-repair enzyme TDP2 in formation of the covalently closed circular DNA persistence reservoir of hepatitis B viruses. Proc Natl Acad Sci U S A 111:E4244-4253. This study identified TDP2 as HBV host factor crucial for cccDNA formation.
Qi Y, Gao Z, Xu G, Peng B, Liu C, Yan H, et al. DNA polymerase κ is a key cellular factor for the formation of covalently closed circular DNA of hepatitis B virus. PLoS Pathog. 2016;12:e1005893.
Kinoshita W, Ogura N, Watashi K, Wakita T. Host factor PRPF31 is involved in cccDNA production in HBV-replicating cells. Biochem Biophys Res Commun. 2017;482:638–44.
Kitamura K, Que L, Shimadu M, Koura M, Ishihara Y, Wakae K, et al. Flap endonuclease 1 is involved in cccDNA formation in the hepatitis B virus. PLoS Pathog. 2018;14:e1007124.
Long Q, Yan R, Hu J, Cai D, Mitra B, Kim ES, et al. The role of host DNA ligases in hepadnavirus covalently closed circular DNA formation. PLoS Pathog. 2017;13:e1006784.
Luo J, Luckenbaugh L, Hu H, Yan Z, Gao L, Hu J (2020) Involvement of host ATR-CHK1 pathway in hepatitis B virus covalently closed circular DNA formation. mBio. 18;11(1):e03423-19.
• Wei L, Ploss A (2020) Core components of DNA lagging strand synthesis machinery are essential for hepatitis B virus cccDNA formation. Nat Microbiol. In press. doi: https://doi.org/10.1038/s41564-020-0678-0. A important study identify the minimal set of factors for cccDNA formation.
Schreiner S, Nassal M (2017) A role for the host DNA damage response in hepatitis B virus cccDNA formation-and beyond? Viruses. 22;9(5):125.
Ji X, Li Z. Medicinal chemistry strategies toward host targeting antiviral agents. Med Res Rev In press doi. 2020. https://doi.org/10.1002/med.21664.
Dandri M. Epigenetic modulation in chronic hepatitis B virus infection. Semin Immunopathol. 2020;42(2):173–85.
Deeks SG. HIV: Shock and kill. Nature. 2012;487:439–40.
Ghosh SK, Perrine SP, Williams RM, Faller DV. Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood. 2012;119:1008–17.
Quasdorff M, Protzer U. Control of hepatitis B virus at the level of transcription. J Viral Hepat. 2010;17:527–36.
Bar-Yishay I, Shaul Y, Shlomai A. Hepatocyte metabolic signalling pathways and regulation of hepatitis B virus expression. Liver Int Off J Int Assoc Study Liver. 2011;31:282–90.
Lucifora J, Arzberger S, Durantel D, Belloni L, Strubin M, Levrero M, et al. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection. J Hepatol. 2011;55:996–1003.
Decorsière A, Mueller H, van Breugel PC, Abdul F, Gerossier L, Beran RK, et al. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531:386–9.
• Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M (2012) IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin invest 122:529–537. This study identified that IFN-α mediates epigenetic repression of HBV cccDNA transcription.
Liu F, Campagna M, Qi Y, Zhao X, Guo F, Xu C, et al. Alpha-interferon suppresses hepadnavirus transcription by altering epigenetic modification of cccDNA minichromosomes. PLoS Pathog. 2013;9:e1003613.
Palumbo GA, Scisciani C, Pediconi N, Lupacchini L, Alfalate D, Guerrieri F, et al. IL6 inhibits HBV transcription by targeting the epigenetic control of the nuclear cccDNA minichromosome. PLoS One. 2015;10:e0142599.
Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatol Baltim Md. 2009;50:1773–82.
Schübeler D. Function and information content of DNA methylation. Nature. 2015;517:321–6.
Zhang Y, Mao R, Yan R, Cai D, Zhang Y, Zhu H, et al. Transcription of hepatitis B virus covalently closed circular DNA is regulated by CpG methylation during chronic infection. PLoS One. 2014;9:e110442.
• Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, Levrero M (2006) Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology 130:823–837. Identification of the enzymatic activities that modulate the acetylation of cccDNA-bound histones as new therapeutic targets for HBV cure.
Yuan Y, Zhao K, Yao Y, Liu C, Chen Y, Li J, et al. HDAC11 restricts HBV replication through epigenetic repression of cccDNA transcription. Antivir Res. 2019;172:104619.
Yu H-B, Jiang H, Cheng S-T, Hu Z-W, Ren J-H, Chen J. AGK2, a SIRT2 inhibitor inhibits hepatitis B virus replication in vitro and in vivo. Int J Med Sci. 2018;15:1356–64.
Gilmore S, Tam D, Dick R, Cheung T, Appleby T, Birkus G, et al. SAT-160 antiviral activityof GS-5801, a liver-targeted prodrug of a lysine demethylase 5 inhibitor, in a hepatitis B virus primary human hepatocyte infection model. J Hepatol. 2017;66:s690–1.
Zhang X, Zhang E, Ma Z, Pei R, Jiang M, Schlaak JF, et al. Modulation of hepatitis B virus replication and hepatocyte differentiation by MicroRNA-1. Hepatol Baltim Md. 2011;53:1476–85.
Feng J, Yang G, Liu Y, Gao Y, Zhao M, Bu Y, et al. LncRNA PCNAP1 modulates hepatitis B virus replication and enhances tumor growth of liver cancer. Theranostics. 2019;9:5227–45.
Hensel KO, Rendon JC, Navas M-C, Rots MG, Postberg J. Virus-host interplay in hepatitis B virus infection and epigenetic treatment strategies. FEBS J. 2017;284:3550–72.
Zlotnick A, Venkatakrishnan B, Tan Z, Lewellyn E, Turner W, Francis S. Core protein: a pleiotropic keystone in the HBV lifecycle. Antivir Res. 2015;121:82–93.
Berke JM, Dehertogh P, Vergauwen K, Van Damme E, Mostmans W, Vandyck K, Pauwels F (2017) Capsid assembly modulators have a dual mechanism of action in primary human hepatocytes infected with hepatitis B virus. Antimicrob Agents Chemother. 25;61(8):e00560-17.
Martinez MG, Villeret F, Testoni B, Zoulim F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int Off J Int Assoc Study Liver. 2020;40(Suppl 1):27–34.
Guo F, Zhao Q, Sheraz M, Cheng J, Qi Y, Su Q, et al. HBV core protein allosteric modulators differentially alter cccDNA biosynthesis from de novo infection and intracellular amplification pathways. PLoS Pathog. 2017;13:e1006658.
Guo Y-H, Li Y-N, Zhao J-R, Zhang J, Yan Z. HBc binds to the CpG islands of HBV cccDNA and promotes an epigenetic permissive state. Epigenetics. 2011;6:720–6.
• Lucifora J, Xia Y, Reisinger F, et al (2014) Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 343:1221–1228. A strategy for elimination of cccDNA by APOBEC3.
Zhang X, Cheng J, Ma J, Hu Z, Wu S, Hwang N, et al. Discovery of novel hepatitis B virus nucleocapsid assembly inhibitors. ACS Infect Dis. 2019;5:759–68.
Wu G, Liu B, Zhang Y, Li J, Arzumanyan A, Clayton MM, et al. Preclinical characterization of GLS4, an inhibitor of hepatitis B virus core particle assembly. Antimicrob Agents Chemother. 2013;57:5344–54.
Weber O, Schlemmer K-H, Hartmann E, Hagelschuer I, Paessens A, Graef E, et al. Inhibition of human hepatitis B virus (HBV) by a novel non-nucleosidic compound in a transgenic mouse model. Antivir Res. 2002;54:69–78.
Brezillon N, Brunelle M-N, Massinet H, Giang E, Lamant C, DaSilva L, et al. Antiviral activity of Bay 41-4109 on hepatitis B virus in humanized Alb-uPA/SCID mice. PLoS One. 2011;6:e25096.
Perni RB, Conway SC, Ladner SK, Zaifert K, Otto MJ, King RW. Phenylpropenamide derivatives as inhibitors of hepatitis B virus replication. Bioorg Med Chem Lett. 2000;10:2687–90.
Delaney WE, Edwards R, Colledge D, Shaw T, Furman P, Painter G, et al. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob Agents Chemother. 2002;46:3057–60.
King RW, Ladner SK, Miller TJ, Zaifert K, Perni RB, Conway SC, et al. Inhibition of human hepatitis B virus replication by AT-61, a phenylpropenamide derivative, alone and in combination with (−)beta-L-2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1998;42:3179–86.
C Campagna MR, Liu F, Mao R, et al (2013) Sulfamoylbenzamide derivatives inhibit the assembly of hepatitis B virus nucleocapsids. J Virol 87:6931–6942.
Berke JM, Dehertogh P, Vergauwen K, Mostmans W, Vandyck K, Raboisson P, Pauwels F (2020) Antiviral properties and mechanism of action studies of the hepatitis B virus capsid assembly modulator JNJ-56136379. Antimicrob Agents Chemother. 21;64(5):e02439-19.
Pei Y, Wang C, Ben H, Wang L, Ma Y, Ma Q, et al. Discovery of new hepatitis B virus capsid assembly modulators by an optimal high-throughput cell-based assay. ACS Infect Dis. 2019;5:778–87.
Na HG, Imran A, Kim K, Han HS, Lee YJ, Kim M-J, et al. Discovery of a new sulfonamide hepatitis B capsid assembly modulator. ACS Med Chem Lett. 2020;11:166–71.
Lahlali T, Berke JM, Vergauwen K, Foca A, Vandyck K, Pauwels F, Zoulim F, Durantel D (2018) Novel potent capsid assembly modulators regulate multiple steps of the hepatitis B virus life cycle. Antimicrob Agents Chemother. 24;62(10):e00835-18.
Amblard F, Boucle S, Bassit L, et al (2020) Novel hepatitis B virus capsid assembly modulator induces potent antiviral responses in vitro and in humanized mice. Antimicrob Agents Chemother. 27;64(2):e01701-19.
Ko C, Bester R, Zhou X, Xu Z, Blossey C, Sacherl J, Vondran FWR, Gao L, Protzer U (2019) A new role for capsid assembly modulators to target mature hepatitis B virus capsids and prevent virus infection. Antimicrob Agents Chemother. 20;64(1):e01440-19.
Zoulim F, Lenz O, Vandenbossche JJ, Talloen W, Verbinnen T, Moscalu I, et al. JNJ-56136379, an HBV capsid assembly modulator, is well-tolerated and has antiviral activity in a phase 1 study of patients with chronic infection. Gastroenterology In press doi. 2020. https://doi.org/10.1053/j.gastro.2020.04.036.
Asselah T, Loureiro D, Boyer N, Mansouri A. Targets and future direct-acting antiviral approaches to achieve hepatitis B virus cure. Lancet Gastroenterol Hepatol. 2019;4:883–92.
Yuen M-F, Agarwal K, Gane EJ, Schwabe C, Ahn SH, Kim DJ, et al. Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: a randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol Hepatol. 2020;5:152–66.
Yuen MF, Gane EJ, Kim DJ, et al (2019) Antiviral activity, safety, and pharmacokinetics of capsid assembly modulator NVR 3-778 in patients with chronic HBV infection. Gastroenterology 156:1392-1403.e7.
Feld J, Lee J, Locarnini S. New targets and possible new therapeutic approaches in the chemotherapy of chronic hepatitis B. Hepatol Baltim Md. 2003;38:545–53.
Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris). 2010;58:258–66.
Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, et al. Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology. 2016;150:194–205.
Boni C, Vecchi A, Rossi M, et al (2018) TLR7 agonist increases responses of hepatitis B virus-specific T cells and natural killer cells in patients with chronic hepatitis B treated with nucleos(t)ide analogues. Gastroenterology 154:1764-1777.e7.
Ma Z, Cao Q, Xiong Y, Zhang E, Lu M (2018) Interaction between hepatitis B virus and toll-like receptors: current status and potential therapeutic use for chronic hepatitis B. Vaccines. 16;6(1):6.
Alonso S, Guerra A-R, Carreira L, Ferrer J-Á, Gutiérrez M-L, Fernandez-Rodriguez CM. Upcoming pharmacological developments in chronic hepatitis B: can we glimpse a cure on the horizon? BMC Gastroenterol. 2017;17:168.
Niu C, Li L, Daffis S, Lucifora J, Bonnin M, Maadadi S, et al. Toll-like receptor 7 agonist GS-9620 induces prolonged inhibition of HBV via a type I interferon-dependent mechanism. J Hepatol. 2018;68:922–31.
Lanford RE, Guerra B, Chavez D, et al (2013) GS-9620, an oral agonist of toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 144:1508–1517, 1517.e1–10.
Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, et al. Sustained efficacy and seroconversion with the toll-like receptor 7 agonist GS-9620 in the woodchuck model of chronic hepatitis B. J Hepatol. 2015;62:1237–45.
Agarwal K, Ahn SH, Elkhashab M, et al (2018) Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J Viral Hepat 25:1331–1340111. Lopatin U (2019) Drugs in the Pipeline for HBV. Clin Liver Dis 23:535–555.
Daffis S, Chamberlain J, Zheng J, Santos R, Rowe W, Mish M, et al. Sustained efficacy and surface antigen seroconversion in the woodchuck model of chronic hepatitis B with the selective toll-like receptor 8 agonist GS-9688. J Hepatol. 2017;66:S692–3.
Bénéchet AP, De Simone G, Di Lucia P, et al. Dynamics and genomic landscape of CD8+ T cells undergoing hepatic priming. Nature. 2019;574:200–5.
Visvanathan K, Skinner NA, Thompson AJV, Riordan SM, Sozzi V, Edwards R, et al. Regulation of toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatol Baltim Md. 2007;45:102–10.
Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, et al. Expression profiles and function of toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol Orlando Fla. 2008;128:400–8.
• Yan W, Wu D, Wang X, Chen T, Lai Q, Zheng Q, Jiang J, Hou J, Han M, Ning Q (2015) Upregulation of NKG2C+ natural killer cells, TLR-2 expression on monocytes and downregulation of regulatory T-cells influence PEG-IFN treatment efficacy in entecavir-suppressed patients with CHB. Antivir Ther 20:591–602. This study characterized the immunological features responsible for treatment responses with pegylated interferon (PEG-IFN)-α2a in entecavir (ETV)-suppressed patients with CHB.
• Visvanathan K, Lang T, Ryan K, et al (2016) Toll-IL1 receptor-mediated innate immune responses vary across HBV genotype and predict treatment response to pegylated-IFN in HBeAg-positive CHB patients. J Viral Hepat 23:170–179. This study suggested clinical differences observed across the CHB spectrum.
Luangsay S, Ait-Goughoulte M, Michelet M, Floriot O, Bonnin M, Gruffaz M, et al. Expression and functionality of toll- and RIG-like receptors in HepaRG cells. J Hepatol. 2015;63:1077–85.
Lucifora J, Bonnin M, Aillot L, Fusil F, Maadadi S, Dimier L, et al. Direct antiviral properties of TLR ligands against HBV replication in immune-competent hepatocytes. Sci Rep. 2018;8:5390.
Verrier ER, Yim S, Heydmann L, et al. Hepatitis B virus evasion from cyclic guanosine monophosphate–adenosine monophosphate synthase sensing in human hepatocytes. Hepatology. 2018;68:1695–709.
Korolowicz KE, Iyer RP, Czerwinski S, Suresh M, Yang J, Padmanabhan S, et al. Antiviral efficacy and host innate immunity associated with SB 9200 treatment in the woodchuck model of chronic hepatitis B. PLoS One. 2016;11:e0161313.
Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64:S71–83.
Rehermann B, Thimme R. Insights from antiviral therapy into immune responses to hepatitis B and C virus infection. Gastroenterology. 2019;156:369–83.
Lang J, Neumann-Haefelin C, Thimme R. Immunological cure of HBV infection. Hepatol Int. 2019;13:113–24.
Gehring AJ, Protzer U. Targeting innate and adaptive immune responses to cure chronic HBV infection. Gastroenterology. 2019;156:325–37.
Bertoletti A, Le Bert N. Immunotherapy for chronic hepatitis B virus infection. Gut Liver. 2018;12:497–507.
•• Hoogeveen RC, Boonstra A (2020) Checkpoint inhibitors and therapeutic vaccines for the treatment of chronic HBV infection. Front Immunol 11:401. A review summarizing current knowledge on checkpoint inhibitors and therapeutic vaccines against HBV infection.
Li J, Bao M, Ge J, Ren S, Zhou T, Qi F, et al. Research progress of therapeutic vaccines for treating chronic hepatitis B. Hum Vaccines Immunother. 2017;13:986–97.
Chinnakannan SK, Cargill TN, Donnison TA, Ansari MA, Sebastian S, Lee LN, et al. The design and development of a multi-HBV antigen encoded in chimpanzee adenoviral and modified vaccinia Ankara viral vectors; a novel therapeutic vaccine strategy against HBV. Vaccines. 2020;8:184.
Kalim M, Iqbal Khan MS, Zhan J. Programmed cell death ligand-1: a dynamic immune checkpoint in cancer therapy. Chem Biol Drug Des In press doi. 2020;95:552–66. https://doi.org/10.1111/cbdd.13677.
Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10:e1003856.
Tseng T-C, Kao J-H. Elimination of hepatitis B: is it a mission possible? BMC Med. 2017;15:53.
Pu D, Yin L, Zhou Y, Li W, Huang L, Cai L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine (Baltimore). 2020;99:e19013.
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–55.
Lake AC. Hepatitis B reactivation in a long-term nonprogressor due to nivolumab therapy. AIDS Lond Engl. 2017;31:2115–8.
Acknowledgements
The authors gratefully acknowledge Dr. Julie Lucifora (CRCL, Inserm Lyon) for her critical reading of the manuscript.
Funding
This work was supported by the Inserm, the University of Strasbourg, the European Union (ERC-2014-AdG-671231-HEPCIR and Horizon 2020 research and innovation programme under grant agreement 667273-HEPCAR), the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) and the French Cancer Agency (ARC IHU201301187). This work has been published under the framework of the LabEx ANR-10-LAB-28 and benefits from a funding from the state managed by the French National Research Agency as part of the Investments for the Future (Investissements d’Avenir) programme. GL is the recipient of an ANRS fellowship (ECTZ86820).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not report conflict of interest. The research organization of T. F. B. has received a research grant in a collaborative research agreement with Janssen Pharmaceuticals.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hepatitis B
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ligat, G., Goto, K., Verrier, E. et al. Targeting Viral cccDNA for Cure of Chronic Hepatitis B. Curr Hepatology Rep 19, 235–244 (2020). https://doi.org/10.1007/s11901-020-00534-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-020-00534-w