Abstract
Despite the discovery of an effective vaccine over 30 years ago, hepatitis B virus (HBV) infection remains a significant cause of global mortality and morbidity affecting over 250 million individuals. Fortunately, many new antiviral agents targeting different steps of the HBV lifecycle are currently being developed marking a true shift in the treatment paradigm. It is hopeful these new therapies will provide a functional and durable cure from HBV infection from a finite duration of treatment.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38.
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55. New World Health Organization estimates on the global prevalence of chronic Hepatitis B infection.
Terrault NA, Bzowej NH, Chang K-M, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. Latest guidelines on the diagnosis and management of chronic hepatitis B infection.
Papatheodoridis G, Buti M, Cornberg M, the European Association For The Study Of The Liver, et al. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85.
Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–61.
Ahn J, Lee HM, Lim JK, Pan CQ, Nguyen MH, Ray Kim W, et al. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US—the ENUMERATE study. Aliment Pharmacol Ther. 2016;43:134–44.
Marcellin P, Gane EJ, Flisiak R, et al. Long term treatment with tenofovir disoproxil fumarate for chronic hepatitis B infection is safe and well tolerated and associated with durable virologic response with no detectable resistance: 8 year results from two phase 3 trials. Boston: 65th Annual Meeting of the American Association for the Study of Liver Diseases; 2014.
Agarwal K, Fung SK, Nguyen TT, et al. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J Hepatol. 2015;62:533–40.
Lai CL, Wong D, Ip P, et al. Profound reduction of HBV covalently closed circular DNA with long-term nucleoside/tide analogue therapy. Boston: 65th Annual Meeting of the American Association for the Study of Liver Diseases; 2014.
Locarnini SA, Yuen L. Molecular genesis of drug-resistant and vaccine-escape HBV mutants. Antivir Ther. 2010;15:451–61.
Lau GK, Piratvisuth T, Luo KX, and the Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–95.
Marcellin P, Lau GK, Bonino F, and the Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–17.
Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135:459–67.
van Zonneveld M, Honkoop P, Hansen BE, et al. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology. 2004;39:804–10.
Janssen HL, van Zonneveld M, Senturk H, and the HBV 99-01 Study Group, the Rotterdam Foundation for Liver Research, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomized trial. Lancet. 2005;365:123–9.
Ning Q, Han M, Sun Y, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol. 2014;61:777–84.
Shi M, Wang RS, Zhang H, et al. Sequential treatment with lamivudine and interferon-alpha monotherapies in hepatitis B e antigen-negative Chinese patients and its suppression of lamivudine-resistant mutations. J Antimicrob Chemother. 2006;58:1031–5.
Chan HL, Leung NW, Hui AY, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med. 2005;142:240–50.
Brouwer WP, Xie Q, Sonneveld MJ, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: a multicenter randomized trial (ARES study). Hepatology. 2015;61:1512–22.
Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48–64.
Claro da Silva T, Polli JE, Swaan PW. The solute carrier family 10 (SLC10): beyond bile acid transport. Mol Aspects Med. 2013;34:252–69.
Eloranta JJ, Jung D, Kullak-Ublick GA. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol Endocrinol. 2006;20:65–79.
Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol. 2005;79:1613–22.
Schulze A, Schieck A, Ni Y, Mier W, Urban S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J Virol. 2010;84:1989–2000.
Petersen J, Dandri M, Mier W, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335–41.
Volz T, Allweiss L, Ben MBarek M, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861–7. First study showing the effectiveness of Myrcludex B inactivating the NTCP receptor and preventing new hepatitis B infection.
Watashi K, Sluder A, Daito T, Matsunaga S, Ryo A, Nagamori S, et al. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter NTCP. Hepatology. 2014;59:1726–37.
Nkongolo S, Ni Y, Lempp FA, et al. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J Hepatol. 2014;60:723–31.
Zimmerman KA, Fischer KP, Joyce MA, Tyrrell DL. Zinc finger proteins designed to specifically target duck hepatitis B virus covalently closed circular DNA inhibit viral transcription in tissue culture. J Virol. 2008;82:8013–21.
Chen J, Zhang W, Lin J, et al. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther. 2014;22(2):303–11.
Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–70. A concise overview of the CRISPR system.
Seeger C, Sohn JA. Targeting hepatitis B virus with CRISPR/Cas9. Mol Ther Nucleic Acids. 2014;3(12):e216. doi:10.1038/mtna.2014.68.
Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res. 2015;118:110–7.
Liu X, Hao R, Chen S, Guo D, Chen Y. Inhibition of hepatitis B virus by CRISPR/Cas9 system via targeting the conserved regions of the viral genome. J Gen Virol. 2015;96:2252–61.
Kennedy EM, Bassit LC, Mueller H, et al. Suppression of hepatitis B virus DNA accumulation in chronically infected cells using a bacterial CRISPR/Cas RNA-guided DNA endonuclease. Virology. 2015;476:196–205.
Belloni L, Allweiss L, Guerrieri F, et al. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122:529–37.
Hannon GJ. RNA interference. Nature. 2002;418:244–51.
Klein C, Bock CT, Wedemeyer H, et al. Inhibition of hepatitis B virus replication in vivo by nucleoside analogues and siRNA. Gastroenterology. 2003;125:9–18.
Shlomai A, Shaul Y. Inhibition of hepatitis B virus expression and replication by RNA interference. Hepatology. 2003;37:764–70.
Yuen M, Chan H, Liu K, et al. ARC-520 produces deep and durable knockdown of viral antigens and DNA in a phase II study in patients with chronic hepatitis B. San Francisco: 66th Annual Meeting of the American Association for the Study of Liver Diseases; 2015.
Gish RD, Yuen MF, Chan HL, et al. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Res 2015;121:97–108.
Feld JJ, Colledge D, Sozzi V, Edwards R, Littlejohn M, Locarnini SA. The phenylpropenamide derivative AT-130 blocks HBV replication at the level of viral RNA packaging. Antiviral Res. 2007;76:168–77.
Deres K, Schroder CH, Paessens A, et al. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science. 2003;299:893–6.
Stray SJ, Bourne CR, Punna S, Lewis WG, Finn MG, Zlotnick A. A heteroaryldihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proc Natl Acad Sci U S A. 2005;102:8138–43.
Wu GY, Zheng XJ, Yin CC, et al. Inhibition of hepatitis B virus replication by Bay 41-4109 and its association with nucleocapsid disassembly. J Chemother. 2008;20:458–67.
Delaney 4th WE, Edwards R, Colledge D, et al. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob Agents Chemother. 2002;46:3057–60.
Liaw S, Brown N, Klumpp K, et al. Phase 1b efficacy and safely of NVR 3-778, a first-in-class HBV core inhibitor, in HBeAg-positive patients with chronic HBV infection. San Francisco: 66th Annual Meeting of the American Association for the Study of Liver Diseases; 2015.
Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754–64.
Korba BE, Montero AB, Farrar K, et al. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008;77:56–63.
Dougherty AM, Guo H, Westby G, et al. A substituted tetrahydro-tetrazolo-pyrimidine is a specific and novel inhibitor of hepatitis B virus surface antigen secretion. Antimicrob Agents Chemother. 2007;51:4427–37.
Yu W, Goddard C, Clearfield E, et al. Design, synthesis, and biological evaluation of triazolo-pyrimidine derivatives as novel inhibitors of hepatitis B virus surface antigen (HBsAg) secretion. J Med Chem. 2011;54:5660–70.
Schadler S, Hildt E. HBV life cycle: entry and morphogenesis. Viruses. 2009;1:185–209.
Siegler VD, Bruss V. Role of transmembrane domains of hepatitis B virus small surface proteins in subviral-particle biogenesis. J Virol. 2013;87:1491–6.
Jansen L, Vaillant A, van Dort K, et al. Serum HBV-RNA levels decline significantly in chronic hepatitis B patients dosed with the nucleic-acid polymer REP 2139-Ca. Vienna: 2015 International Liver Congress: 50th Annual Meeting of the European Association for the Study of the Liver (EASL); 2015.
Hopkins S, DiMassimo B, Rusnak P, et al. The cyclophilin inhibitor SCY-635 suppresses viral replication and induces endogenous interferons in patients with chronic HCV genotype 1 infection. J Hepatol. 2012;57:47–54.
Hopkins S, Bobardt M, Chatterji U, Garcia-Rivera JA, Lim P, Gallay PA. The cyclophilin inhibitor SCY-635 disrupts hepatitis C virus NS5A-cyclophilin A complexes. Antimicrob Agents Chemother. 2012;56:3888–97.
Phillips S, Chokshi S, Chatterji U, et al. Alisporivir inhibition of hepatocyte cyclophilins reduces HBV replication and hepatitis B surface antigen production. Gastroenterology. 2015;148:403–14.
Acknowledgments
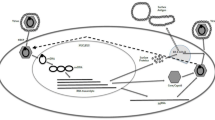
We would like to thank David Smookler for his help with the illustration of the hepatitis B virus lifecycle.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
MB declares that he has no conflict of interest. HLAJ reports research support, consulting, or speaking fees from Gilead, Novartis, Roche, Merck, AbbVie, Bristol-Myers Squibb, Arbutus, Janssen, MedImmune, and Ionis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Additional information
This article is part of the Topical Collection on Hepatitis B
Rights and permissions
About this article
Cite this article
Brahmania, M., Janssen, H.L.A. Future Therapy for HBV: Role of Cell Cycle Inhibitors. Curr Hepatology Rep 15, 245–251 (2016). https://doi.org/10.1007/s11901-016-0313-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-016-0313-y